N-(2,4,6-Tribromophenyl)maleimide CAS#: 59789-51-4; ChemWhat Code: 898326
Identification
| Product Name | N-(2,4,6-Tribromophenyl)maleimide |
| IUPAC Name | 1-(2,4,6-tribromophenyl)pyrrole-2,5-dione |
| Molecular Structure | 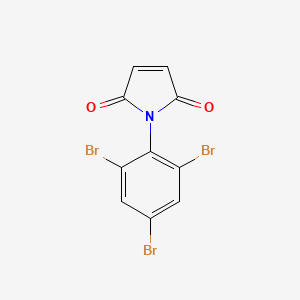 |
| CAS Registry Number | 59789-51-4 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | N-(2,4,6-tribromophenyl)maleimideN-(2,4,6-Tribromphenyl)-maleinimid |
| Molecular Formula | C10H4Br3NO2 |
| Molecular Weight | 409.86 |
| InChI | InChI=1S/C10H4Br3NO2/c11-5-3-6(12)10(7(13)4-5)14-8(15)1-2-9(14)16/h1-4H |
| InChI Key | ONEIBTGSNPDDSB-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=O)N(C1=O)C2=C(C=C(C=C2Br)Br)Br |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP3498694 | NEW BENZAMIDE DERIVATIVES AS PPAR-GAMMA MODULATORS | 2019 |
| WO2019/126730 | CHROMENOPYRIDINE DERIVATIVES AS PHOSPHATIDYLINOSITOL PHOSPHATE KINASE INHIBITORS | 2019 |
| US2018/230157 | PYRROLO[1,2-b]PYRIDAZINE DERIVATIVES | 2018 |
| WO2018/169373 | PYRROLOTRIAZINE DERIVATIVES AS KINASE INHIBITOR | 2018 |
| WO2018/203194 | DIAZABICYCLOOCTANE DERIVATIVES COMPRISING A QUATERNERY AMMONIUM GROUP FOR USE AS ANTIBACTERIAL AGENTS | 2018 |
Physical Data
| Appearance | Light yellow powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C |
| 142.5 |
| 142 – 145 |
Spectra
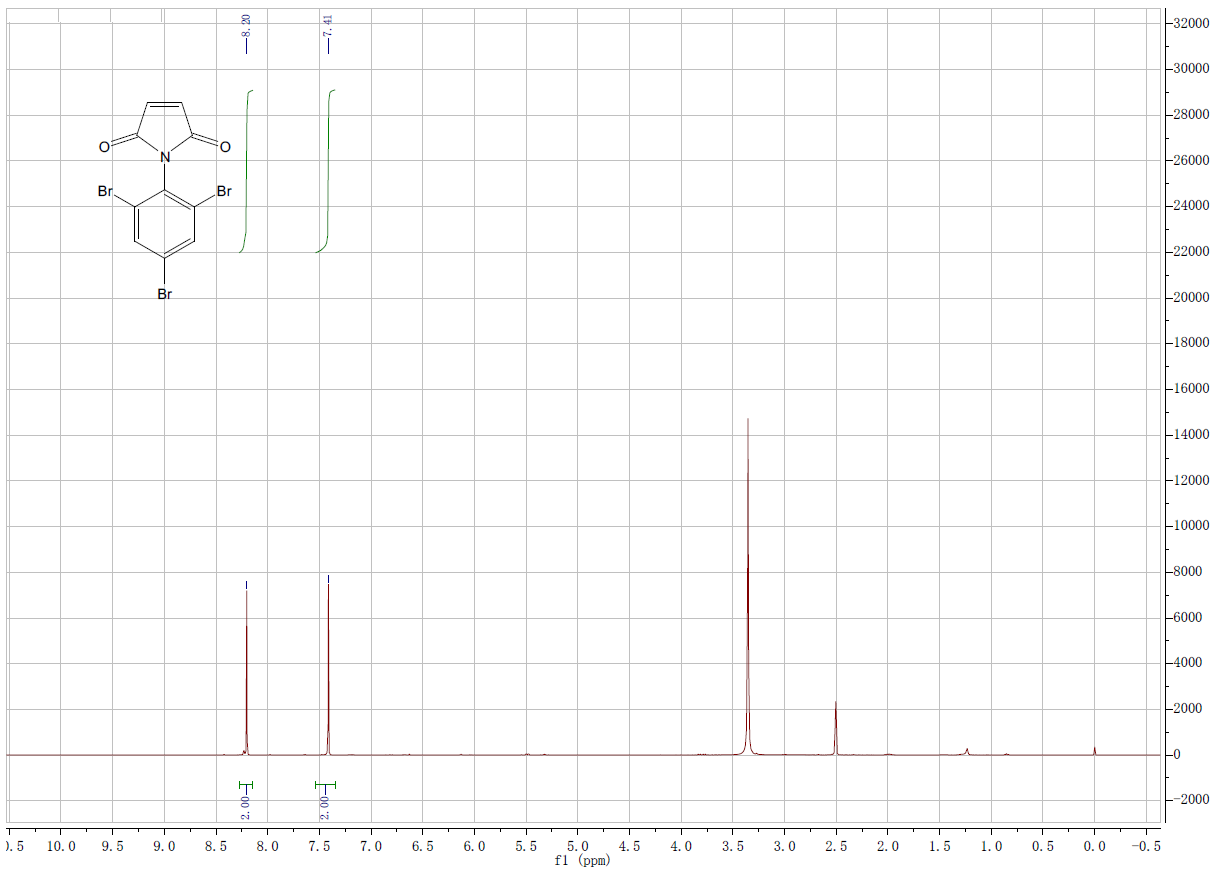
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Coupling Nuclei | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | CDCl3 | 300 | |
| Chemical shifts | 13C | CDCl3 | 75 | |
| 13C | 1H | CDCl3 | 75 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| IR |
Route of Synthesis (ROS)

| Conditions | Yield |
| With hydrogen In ethyl acetate at 20℃; under 7600.51 Torr; for 6h; Autoclave; | 99% |
| With 0.2C27H36N2*Pt; hydrogen In tetrahydrofuran at 60℃; under 3000.3 Torr; for 5h; chemoselective reaction; | 99% |
| With hydrogen In ethyl acetate under 760.051 Torr; for 2h; Heating; Flow reactor; Green chemistry; | 99% |
| With hydrogen; triethylamine In ethanol; water at 110℃; under 30003 Torr; for 24h; Autoclave; | 98% |
| With hydrogen In 2-methyltetrahydrofuran; water at 40℃; under 15001.5 Torr; for 24h; chemoselective reaction; | 98% |
| With sodium tetrahydroborate In water at 20℃; for 1.5h; chemoselective reaction; Experimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | 97% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 409.859 |
| logP | 3.426 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.38 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Can be used in aerospace composite materials (high temperature heat resistance); PCB electronic insulation materials (good flame retardancy); 3D printing photosensitive resins (high reactivity); high-performance coatings, adhesives (reactive structures) |
| High-performance polymer additives or monomers Used to synthesize special thermosetting resins or high-temperature resistant polymers, such as polyimide and maleimide copolymers; its maleimide structure can undergo addition polymerization, free radical polymerization or Diels-Alder reaction with other monomers; the aromatic tribromo substitution group gives the product flame retardancy and thermal stability, suitable for use in high-performance materials such as aviation, electronics, and insulation. |
| Flame retardant intermediates or synergists The Br (bromine) element on the aromatic ring has excellent flame retardant effect; this product can be used as a reactive flame retardant intermediate, embedded in the main chain or side chain of the polymer to improve the flame retardant efficiency and reduce migration; it is often used for efficient flame retardant modification of polyester, polyamide, epoxy resin and other systems. |
| Electronic material intermediates N-aryl maleimide compounds have excellent electronic properties and are widely used in: organic semiconductors, optoelectronic functional materials, photoresists and other functional polymer monomers. After tribromo substitution, it can be further derivatized, such as participating in Suzuki or Stille coupling to construct a π conjugated system. |
| Photosensitizer/crosslinker Maleimide groups have good electron affinity and are often used in photo-initiated crosslinking reactions or UV curing systems. They can be rapidly photocrosslinked with thiols and alkenes to form a crosslinked network structure, which is used in photoresists, adhesives and other fields. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |