N-(2,4,6-Trichlorophenyl)maleimide CAS#: 13167-25-4; ChemWhat Code: 133237
Identification
| Product Name | N-(2,4,6-Trichlorophenyl)maleimide |
| IUPAC Name | pyridin-3-amine |
| Molecular Structure | 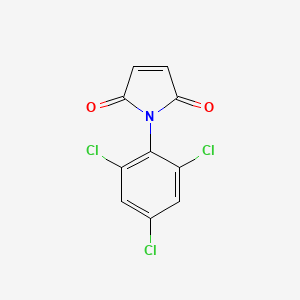 |
| CAS Registry Number | 13167-25-4 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | N-(2,4,6-trichlorophenyl)maleimide |
| Molecular Formula | C10H4Cl3NO2 |
| Molecular Weight | 276.5 |
| InChI | InChI=1S/C10H4Cl3NO2/c11-5-3-6(12)10(7(13)4-5)14-8(15)1-2-9(14)16/h1-4H |
| InChI Key | VHZJMAJCUAWIHV-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=O)N(C1=O)C2=C(C=C(C=C2Cl)Cl)Cl |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN115353477 | Preparation method of bis-selenomaleimide compound | 2022 |
| US2003/191091 | Use of riboflavin and flavin derivatives as chitinase inhibitors | 2003 |
Physical Data
| Appearance | Light yellow powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 134 |
Spectra
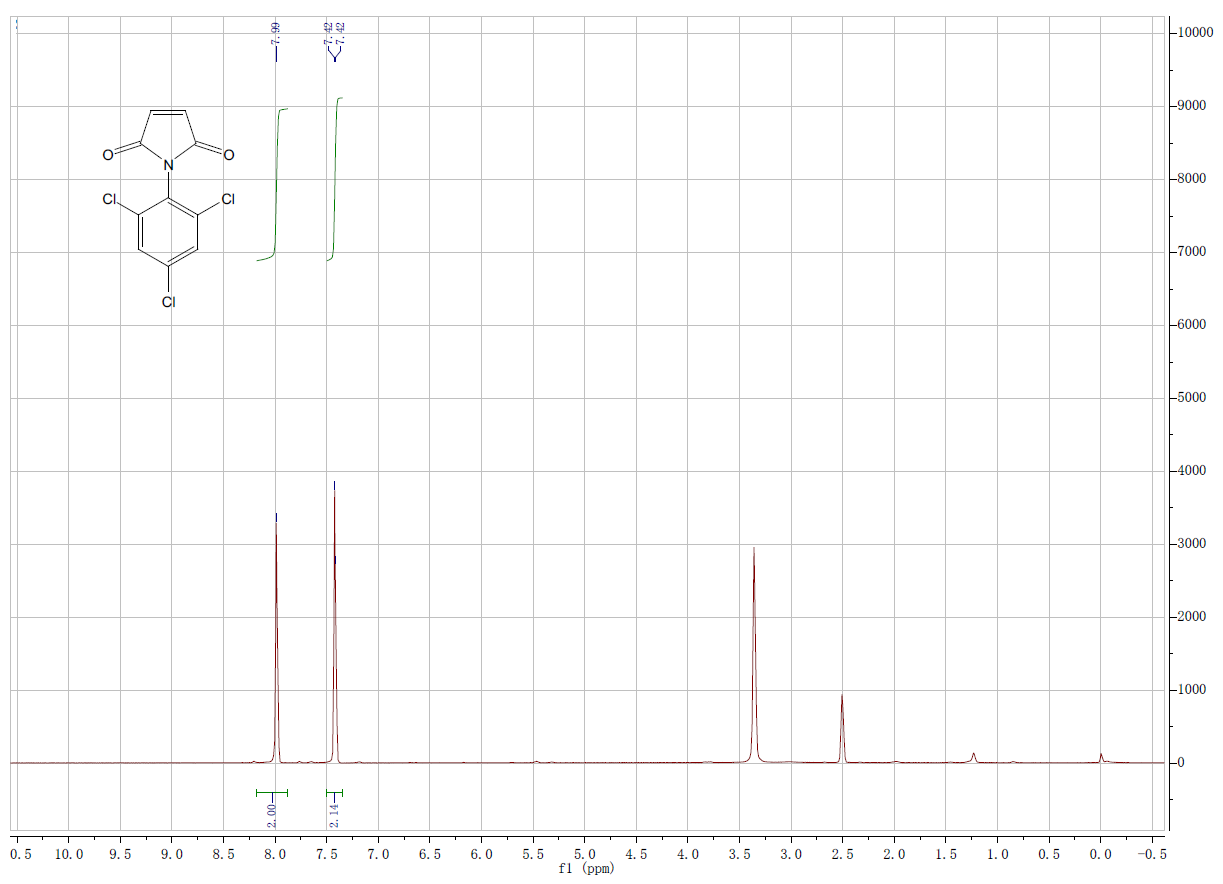
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H |
| Description (IR Spectroscopy) |
| Bands, Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver(l) oxide In 2,2,2-trifluoroethanol at 80℃; for 3h; | 61% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, and P362+P364 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 276.506 |
| logP | 2.898 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.38 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Ingredient of an antifouling paint |
| High-performance polymer intermediates This compound is often used to prepare heat-resistant and insulating polymers, such as polyimide and maleimide copolymers; it can be used as a comonomer of thermosetting resins to give the material high strength and thermal oxygen stability; chlorine atoms enhance the flame retardancy and chemical corrosion resistance of the material. Used in aerospace composites; high-end electronic/electrical insulation materials; high-temperature adhesives or coatings. |
| Photosensitive materials and crosslinkers The maleimide structure makes it suitable for UV curing and thermal crosslinking systems; the structure has high rigidity and is often used as a monomer for photoresists, liquid crystal orienting agents or photosensitive polymers; it can be used in conjunction with photoinitiators for electronic packaging materials. |
| Pharmaceutical or pesticide intermediates (potential) Chloroaryl groups have certain antibacterial, antifungal or insecticidal properties, and can theoretically be used as precursors for certain pesticide or pharmaceutical active molecules; maleimide groups are often used to synthesize heterocyclic drug candidates with diverse structures (such as the parent nucleus of anticancer and antiviral drugs); |
| Special additives or functional coating materials Trichlorosubstituted groups make them hydrophobic and antimicrobial; they can be used to synthesize antibacterial polymer coatings, functionalize the surface of medical devices, and modify membrane materials; they are suitable for coating systems with solvent resistance, scratch resistance, and anti-aging functions. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |