N,N’-Bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide CAS#: 42774-15-2; ChemWhat Code: 634605
Identification
| Product Name | N,N’-Bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide |
| IUPAC Name | 1-N,3-N-bis(2,2,6,6-tetramethylpiperidin-4-yl)benzene-1,3-dicarboxamide |
| Molecular Structure | 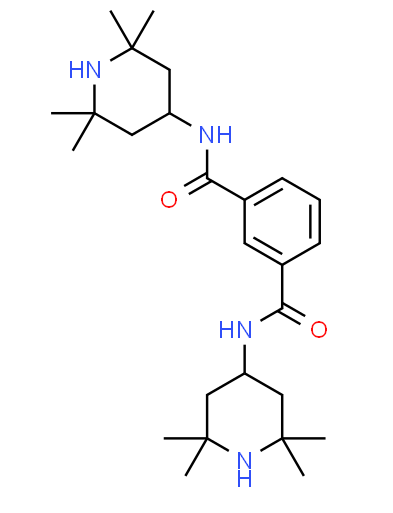 |
| CAS Registry Number | 42774-15-2 |
| Synonyms | N,N’-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide, N,N’-bis-(2,2,6,6-tetramethylpiperidin-4-yl)isophthalamide |
| Molecular Formula | C26H42N4O2 |
| Molecular Weight | 442.65 |
| InChI | InChI=1S/C26H42N4O2/c1-23(2)13-19(14-24(3,4)29-23)27-21(31)17-10-9-11-18(12-17)22(32)28-20-15-25(5,6)30-26(7,8)16-20/h9-12,19-20,29-30H,13-16H2,1-8H3,(H,27,31)(H,28,32) |
| InChI Key | OYNOCRWQLLIRON-UHFFFAOYSA-N |
| Canonical SMILES | CC1(CC(CC(N1)(C)C)NC(=O)C2=CC(=CC=C2)C(=O)NC3CC(NC(C3)(C)C)(C)C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN103554009 | A nylon multi-functional process for the preparation of stabilizers (by machine translation) | 2016 |
| WO2004/16591 | PROCESS FOR THE PREPARATION OF STABILIZERS FOR POLYMERS | 2004 |
Physical Data
| Appearance | White crystalline powder |
| Density | 1.09±0.1 g/cm3 |
| Melting Point, °C | Comment (Melting Point) |
| 272 | |
| 278 – 280 | |
| 270 – 275 | |
| 271.3 – 272 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | |||
| Chemical shifts, Spectrum | 1H | d(4)-methanol | 500 | |
| Linewidth of NMR absorption | 13C | d(4)-methanol |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands, Spectrum |
Route of Synthesis (ROS)
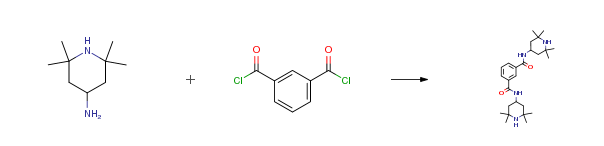
| Conditions | Yield |
| With aluminum (III) chloride; sodium hydroxide In toluene at 110℃; Concentration; Experimental Procedure the electric stirrer, reflux condenser and a thermometer in four of the reaction bottle, adding m-phthaloyl chloride 20.3g, 2, 2, 6, 6-tetramethyl-4-PIPERIDYLAMINES 36g, solvent 60g, catalyst 1.5g, a reflux condensing tube, thermometer, the agitator is started, the temperature is increased to 110 °C time, start timing, the reaction time is 4.5-10h, after the reaction, under strong stirring, slowly into the reactant in aqueous solution of alkali, to remove the unreacted m-phthaloyl chloride, then return water, lowering the temperature to 15-20 °C to crystallization, filtration can get light stabilizer N, N, -bis-(2, 2, 6, 6-tetramethyl-4-piperidinyl) isophthalamide, light stabilizer N, N, -bis-(2, 2, 6, 6-tetramethyl-4-piperidinyl) isophthalamide the yield reaches 97.38percent, melting point is 270-275°C. | 97.38% |
| With sodium hydroxide In water; isopropyl alcohol at 30 – 130℃; under 1125.11 – 2475.25 Torr; Alkaline aqueous solution; Experimental Procedure In a 4-necked 2 I flask with stirrer, dropping funnel, thermometer and pH electrode 150.5 [G] of 2,2, 6, 6-Tetramethylpiperidin-4-yl-amine (98.7 percent; 0.95 mole) and 85.2 [G] of 50percent [NAOH] solution (1.07 mole) are added to a mixture of 470.0 [G] of isopropanol and 260.0 g of demin. water. Under stirring 102.1 [G] of molten isophthalic acid chloride (99.4 percent; 0.50 mole) are added. The temperature of the reaction mixture is held at [30°C] during dosage of IPC by cooling with an ice bath. The reaction mixture is stirred for another 1 hour under the same conditions (temperature/pH-control). A white suspension is being formed during this reaction phase. The reaction mixture is transferred to a 3 l laboratory autoclave with stirrer and internal thermometer and the mixture is heated to a temperature of [TI] [100°C.] The resulting pressure of the system is about 1.5 bar and the solid is being dissolved completely. Two liquid phases are being formed: – Lower aqueous phase containing salts and aqueous [NAOH] and some isopropanole – Upper organic phase containing desired product solved in isopropanole ; The lower phase is removed under pressure and 950.0 [G] of demin. water is added to the reaction mixture. The mixture is further heated up to TI = [130° C] and a corresponding system pressure of about PI = 3.3 bar until all of the solid has completely dissolved. The suspension is allowed to cool down to ambient temperature TI [ 30°C.] The resulting white suspension is passed through a filter aggregate with metal screen and the reaction product is washed with 715.0 [G] of demin. water to remove chloride and other impurities. The reaction product is heated in a vacuum drying oven until constancy of weight. The yield of reaction product of general formula [(I)] with Ri = methyl, and R2 = H is 200.0 g or 95.3 percent of theoretical value (yield based on minor component TAD). | 95.3% |
| With sodium hydroxide In chloroform Solvent; Reflux; | 94.9% |
| With sodium hydroxide In water at 7 – 25℃; for 3.5h; Alkaline aqueous solution; | 83.6% |
| With sodium hydroxide In water; isopropyl alcohol at 100℃; | 200g |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
Use Pattern
| N,N’-Bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide CAS#: 42774-15-2 can be used for polymers/polymer applications |
| N,N’-Bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide CAS#: 42774-15-2 can be used as a nylon multi-functional stabilizer |
| Stabilizer for polymers |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

