p-Hydroxybenzaldehyde CAS#: 123-08-0; ChemWhat Code: 28220
Identification
| Product Name | p-Hydroxybenzaldehyde |
| IUPAC Name | 4-hydroxybenzaldehyde |
| Molecular Structure | 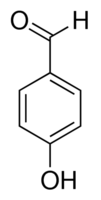 |
| CAS Registry Number | 123-08-0 |
| EINECS Number | 204-599-1 |
| MDL Number | MFCD00006939 |
| Beilstein Registry Number | 471352 |
| Synonyms | 4-hydroxy-benzaldehyde, p-hydroxylbenzaldehyde, 4-hydroxybenzaldehyde;CAS No.: 123-08-0; CAS Number: 123-08-0 |
| Molecular Formula | HOC6H4CHO |
| Molecular Weight | 122.12 |
| InChI | InChI=1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H |
| InChI Key | RGHHSNMVTDWUBI-UHFFFAOYSA-N |
| Canonical SMILES | c1cc(ccc1C=O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110668921 | Method for preparing alcohol and phenol by aerobic hydroxylation reaction of boric acid derivative under non-light catalyst condition (by machine translation) | 2020 |
| CN110818537 | One-pot synthesis method of phenolic oxygen enyne ether compound (by machine translation) | 2020 |
| US2009/118514 | PROCESSES FOR PREPARING PIOGLITAZONE AND ITS PHARMACEUTICALLY ACCEPTABLE SALTS | 2009 |
| US2011/3397 | Solvatochromic functional monomer and the use thereof for chemosensing by solvatochromic molecular imprinting | 2011 |
Physical Data
| Appearance | Creamish, Yellow to Tan color powder |
| Solubility | 13.8g/l |
| Refractive index | 1.5105 (estimate) |
| Sensitivity | Air Sensitive |
| Melting Point, °C |
| 112 |
| 110 – 113 |
| 112.3 – 114.3 |
| 116 – 118 |
| 117 – 119 |
| Boiling Point, °C |
| 116 |
| 310 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.5628 | 589 | 20 |
| 1.57055 | 656.3 | 130 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.288 | 17.84 | |
| 1.134 | 131 | |
| 1.086 | 4 | 191.6 |
| 1.0914 | 4 | 174.8 |
| 1.1119 | 4 | 150.4 |
| 1.1171 | 4 | 130.4 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| IR spectrum of the complex | CCl4 | diethylene glycol monohexyl ether | |
| Stability constant of the complex with … | dibutyl ether | 21 | H2O |
| Spectrum of the complex |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 13C | chloroform-d1 | ||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 | |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Bands | |
| Bands, Spectrum | potassium bromide |
| ATR (attenuated total reflectance), Bands | neat (no solvent, solid phase) |
| Bands | tetrachloromethane |
| Mid IR (MIR), Bands | potassium bromide |
| in KBr |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum |
| electrospray ionisation (ESI), spectrum |
| electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), spectrum |
| gas chromatography mass spectrometry (GCMS), spectrum |
| time-of-flight mass spectra (TOFMS), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | dimethyl sulfoxide | 284, 354 |
| Spectrum | ethanol | 284 |
| Absorption maxima | methanol | 220, 284 |
| Absorption maxima | aq. NaOH | 240, 330 |
| UV/VIS | ||
| Absorption maxima | hexane | 265.5, 281, 288 |
| Description (Raman Spectroscopy) | Comment (Raman Spectroscopy) |
| Raman | |
| Spectrum | |
| Bands | CCl4; 1700-1550 cmE-1 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogenchloride In acetic acid at 25 – 30℃; for 50h; | 95% |
| With hydrogenchloride In methanol; water at 20℃; for 24h; | 95% |
| With nanometasilica disulfuric acid In water for 0.416667h; Reflux; Green chemistry; | 92% |
| With molybdenum(V) chloride In neat (no solvent) for 0.2h; Claisen-Schmidt Condensation; Microwave irradiation; Green chemistry; | 91% |
| With sulfonated montmorillonite/poly(ethylene glycol) nanocomposite [(Mt/PEG)-SO3H] In neat (no solvent) at 20℃; for 0.783333h; | 90% |
| With carbon nanocage material CKT-3(A) at 70℃; for 0.833333h; | 84.4% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 291249 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 122.123 |
| logP | 1.312 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.3 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 6.4 | Kic | = | 0.4 | µM | |
| 6.3 | IC50 | = | 0.6 | µM | |
| 6.22 | Ki (inhibition constant) | = | 0.6 | µM | |
| 6 | Kd (dissociation constant) | 1 | µM | ||
| 5.98 | activity percentage | 116.08 | % | anti-neuroinflammatory agent | |
| 5.96 | Kd (dissociation constant) | 1.1 | µM | ||
| 5.8 | Kd (dissociation constant) | 1.6 | µM | ||
| 5.72 | Kd (dissociation constant) | 1.9 | µM | ||
| 5.48 | IC50(Radical Scavenging Activity) | = | 3.3 | µM |
| Quantitative Results | ||
| 1 of 10 | Effect | antimicrobial agent |
| Biological material | Enterococcus faecalis | |
| Assay Description | Bioassay : Microbicidal Test Agar Dilution Method As microbicidal test method for trans-p-coumaric acid, 3-hydroxypyridine, p-hydroxybenzaldehyde and vanillin, the agar dilution method was employed. In agar dilution method, a solution in which a sample is dissolved is serially diluted several times, and each | |
| 2 of 10 | Effect | sporacidal agent |
| Assay Description | Target : ATCC 16404 of Aspergillus niger Bioassay : Example 1Sporicidal effect of test compounds in waterAspergillus niger ATCC 16404 spores are added to water to obtain a density of 3 x 105 spores / ml. In order to prepare the spores, the test strain is grown for 5 days on potato dextrose agar at room temperature. The spores are harvested | |
| Results | 0.1 % test compound; spores >1.2E5 ml; reduction factor <2; no significant reduction of spore conunts is achieved with title compound; reduction factor 7d fungal spores 1 | |
| 3 of 10 | Effect | antimicrobial agent |
| Biological material | Enterococcus faecalis | |
| Assay Description | Effect : microbicidal Microbicidal Test Agar Dilution Method As microbicidal test method for trans-p-coumaric acid, 3-hydroxypyridine, p-hydroxybenzaldehyde and vanillin, the agar dilution method was employed. In agar dilution method, a solution in which a sample is dissolved is serially diluted several times, and each | |
| 4 of 10 | Effect | sporacidal agent |
| Biological material | Aspergillus niger | |
| Assay Description | Effect : sporicidal Bioassay : ATCC 16404 Example 1Sporicidal effect of test compounds in waterAspergillus niger ATCC 16404 spores are added to water to obtain a density of 3 x 105 spores / ml. In order to prepare the spores, the test strain is grown for 5 days on potato dextrose agar at room temperature. The spores are harvested | |
| Results | 0.1 % test compound; spores >1.2E5 ml; reduction factor <2; no significant reduction of spore conunts is achieved with title compound; reduction factor 7d fungal spores 1 | |
| 5 of 10 | Effect | radical scavenging |
| Assay Description | Radical scavenging activity of the compound was determined | |
| Results | NA = Not active, radical scavenging less than 20% at concentration of 5 mg/mL | |
| 6 of 10 | Effect | inhibitory activity |
| Target | Alpha-glucosidase (gene aglA):Wild | |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory concentration of the compound against Alpha-glucosidase | |
| Results | NT = not tested | |
| 7 of 10 | Effect | antiproliferative agent |
| Biological material | A-549 cell line | |
| Assay Description | Growth inhibitory concentration of compound against human solid tumor non-small cell lung A549 cell line upon incubation for 48 hrs at 37 degree C in presence of 2 mM L-glutamine by using SULFORHODAMINE B (SRB) ASSAY | |
| Measurement | SI50 | |
| 8 of 10 | Effect | inhibitory activity |
| Substance action on target | Inhibitor | |
| Assay Description | Percent inhibition of Lipid peroxidation at 0.74 mM compound upon incubation in 0.2 M phosphate buffer, pH 7.0 for 3 mins at RT by using ferric thiocyanate method | |
| Measurement | Cell viability | |
| 9 of 10 | Effect | radical scavenging |
| Assay Description | Hydrogen peroxide scavenging activity of 100 uM compound upon incubation for 0.1 M phosphate buffer, pH 7.4 for 20 mins at 37 degree C | |
| Results | No activity | |
| 10 of 10 | Effect | inhibitory activity |
| Target | Alpha-glucosidase (gene aglA):Wild | |
| Substance action on target | Inhibitor | |
| Assay Description | Percent inhibition of ALPHA-GLUCOSIDASE upon incubation at 25 degree C in 0.1 M phosphate buffer, pH 6.8 with 500 uM compound concentration using p-NITROPHENYL-ALPHA-D-GLUCOPYRANOSIDE as substrate | |
| Results | No inhibition |
| Use Pattern |
| p-Hydroxybenzaldehyde CAS#: 123-08-0 as Cosmetics/dental/toilet |
| p-Hydroxybenzaldehyde CAS#: 123-08-0 as Detergents/disinfections |
| p-Hydroxybenzaldehyde CAS#: 123-08-0 as Food/food additives |
| p-Hydroxybenzaldehyde CAS#: 123-08-0 as Pharmaceuticals |
| an antimicrobial mixture in preservation of cosmetic compositions in combination with at least one monocyclic sesquiterpene alcohol and 4-hydroxyacetophenone |
| an antimicrobial mixture in preservation of cosmetic compositions in combination with at least one monocyclic sesquiterpene alcohol and glyceryl caprate |
| an antimicrobial mixture in preservation of detergent compositions in combination with at least one monocyclic sesquiterpene alcohol and cocamidopropyl PG-dimonium chloride phosphate |
| an antimicrobial mixture in preventing or treating a pharmaceutical composition against microbial attack in combination with at least one monocyclic sesquiterpene alcohol and cocamidopropyl PG-dimonium chloride phosphate |
| intermediate for synthesis of resveratrol |
| melanin tumor |
| prevent and treat human body hyperpigmentation diseases caused by melanin synthesis abnormity |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |