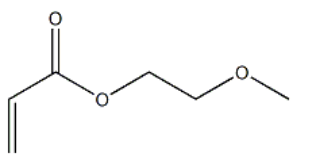
POLY(ETHYLENE GLYCOL) METHYL ETHER ACRYLATE CAS#: 32171-39-4; ChemWhat Code: 51523
Identification
Physical Data
| Appearance | Colorless transparent liquid |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 56 | 12 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.0131 | 4 | 20 |
Spectra
| Description (IR Spectroscopy) |
| Spectrum |
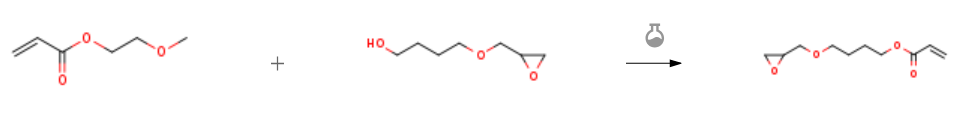
Route of Synthesis (ROS)
| Conditions | Yield |
| With tetrabutoxytitanium; 4-methoxy-phenol In o-xylene at 80 – 90℃; under 75.0075 Torr; Experimental Procedure In a 500 ml four-necked flask equipped with a distillation apparatus, a thermometer, and a stirrer,1,4-butanediol monoglycidyl ether (hereinafter referred to as “14 BDMGE”100.0 g (purity: 93.7%, 0.64 mol in terms of purity)),125.1 g (0.96 mol) of 2-methoxyethyl acrylate,200.0 g of o-xylene and 0.03 g of MEHQ were added to prepare a raw material mixture solution(Total 425.1 g) were prepared.When the moisture content of this raw material mixture liquid was measured with a Karl Fischer moisture meter, it was 503 ppm, and the total amount of moisture contained was 0.21 g (0.012 mol). To the above raw material mixture solution, 4.4 g (0.013 mol) of TBT was added,While heating the solution under reduced pressure while reducing the pressure to 100 hPa, transesterification reaction was carried out at a reaction solution temperature of 80 to 90 ° C. while o-xylene and produced 2-methoxyethanol were distilled out of the reaction system.The temperature of the distillation gas was 57 to 74 ° C.Finally, 169.9 g of the fraction was withdrawn in 6 hours and the reaction was terminated to obtain 255.2 g of a reaction solution containing 4 HBAGE (46.3 wt%).The purity-converted quantitative yield of 4 HBAGE is 92.0%, and the molar ratio of the total amount of moisture in this reaction system is 0.9 times the TBT used. To the above reaction solution, 140.0 g of water was added, heated to 60 ° C. under normal pressure with stirring, and heated and hydrolyzed at 60 ° C. for 1 hour as it was. The reaction solution was cooled and suction filtered using a filter equipped with Celite to remove insoluble catalyst. The filtrate was separated into an organic layer and an aqueous layer. The organic layer containing 4 HBAGE was concentrated under reduced pressure using a rotary evaporator to obtain 130.8 g of 4 HBAGE crude product (purity 94.0%). The purity-converted quantitative yield of 4 HBAGE was 95.8%, and the content of titanium derived from TBT used as a catalyst was 2 ppm or less.50.0 g of the above 4 HBAGE crude product (130.8 g) was fractionated, 0.08 g of CBC was added thereto, and purification was carried out by simple distillation under reduced pressure. As a result, high purity 4 HBAGE (purity 96.9%) was obtained.The purity-converted quantitative yield of 4 HBAGE is 83.7% in terms of the consistent yield from the transesterification reaction. Conditions at the time of fractional distillation were 0.4 to 0.6 hPa in the degree of vacuum, 103 to 114 ° C. in the bottom liquid temperature, 93 to 95 ° C. in the distillation gas temperature, about 1 hour in distillation time there were. No polymer was observed in the distillation residue and in the distillation system. | 83.7% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H226 (89.4%): Flammable liquid and vapor [Warning Flammable liquids] H302 (89.4%): Harmful if swallowed [Warning Acute toxicity, oral] H311+H331 (73.1%): Toxic in contact with skin or if inhaled. [Danger Acute toxicity, dermal; acute toxicity, inhalation] H311 (89.4%): Toxic in contact with skin [Danger Acute toxicity, dermal] H314 (74%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H315 (25%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (77.9%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (37.5%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (25%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H331 (78.8%): Toxic if inhaled [Danger Acute toxicity, inhalation] H332 (10.6%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (11.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H341 (19.2%): Suspected of causing genetic defects [Warning Germ cell mutagenicity] H360 (57.7%): May damage fertility or the unborn child [Danger Reproductive toxicity] H360FD (17.3%): May damage fertility; May damage the unborn child [Danger Reproductive toxicity] H373 (63.5%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H411 (15.4%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] H412 (72.1%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P203, P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P264+P265, P270, P271, P272, P273, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P303+P361+P353, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P318, P319, P321, P330, P332+P317, P333+P317, P337+P317, P361+P364, P362+P364, P363, P370+P378, P391, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store at room temperature for long time, sealed and keep in a dry place. |
| HS Code | |
| Storage | Store at room temperature for long time, sealed and keep in a dry place. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 130.144 |
| logP | -0.343 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 35.53 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| MPEG-AC CAS 32171-39-4 finds extensive applications in UV-curable systems and related fields. It is widely used as a key raw material in high-performance UV-curable adhesives, particularly for laminated glass, flexible displays, and optical device bonding. In UV-curable systems, it acts as a modifier to adjust curing speed, flexibility, and adhesion performance, optimizing the physical and chemical properties of the final product. It serves as a monomer in polymerization reactions for the synthesis of specialty acrylic resins or cyclohexyl-based compounds. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |