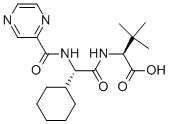
Stage #1: (S)-methyl 2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoate With sodium hydroxide In tetrahydrofuran; methanol at 0 – 20℃;
Stage #2: With potassium hydrogensulfate In water pH=3.5;
Experimental Procedure
A solution of 1 M NaOH (0.94 ml, 0.94 mmol) was added to a solution of 11 (0.31 g, 0.78 mmol) in THF (3 ml) at 0°C. MeOH was added to the formed suspension, to give a clear and colourless solution. The reaction mixture was stirred overnight at room temperature, followed by concentration in vacuo. The pH of this aqueous layer was set to 3.5 with 1 M KHSO4 and subsequently extracted with EtOAc (2 χ 10ml). The mixture was dried with Na2S04, filtered, and concentrated in vacuo, to give 2 (0.28 g, 0.75 mmol, 95%) as a white solid.[a f° = +21.7 (c= 1.015, CHC13); *H NMR (250.13 MHz, CDC13): δ = 9.39 (d, J= 1.3Hz, 1H), 8.77 (d, J = 2.5 Hz, 1H), 8.57 (dd, J = 1.5, 2.5 Hz, 1H), 8.35 (d, J = 9 Hz, 1H), 6.70 (d, J = 9.0 Hz, 1H), 4.45 (t, J = 8.8 Hz, 1H), 4.42 (d, J = 9.2 Hz, 1H), 1.94 (m, 1H), 1.71 (m, 5H), 1.20 (m, 5H), 1.01 (s, 9H); 13C NMR (62.90 MHz, CDCI3): δ = 173.4 (C), 170.5 (C), 163.3 (C), 147.4 (CH), 144.4 (CH), 144.2 (C), 142.8 (CH), 58.4 (CH), 51.9 (CH), 40.4 (CH), 34.7 (C), 29.8 (CH2), 28.6 (CH2), 26.6 (CH3), 25.8 (CH2); IR (neat): v„(cm ) = 3335 (w), 2930 (m), 1726 (m), 1663 (s), 1514 (s); HRMS (ESI, 4500 V): m/z calc. for Ci9H29N404Na+ ([M + Na]+) 399.2003, found 399.2013. | 95% |
With water; sodium hydroxide In methanol at 40 – 45℃; for 24h; Inert atmosphere;
Experimental Procedure
3 Synthesis of (S)-2-((S)-2-cyclohexyl-2-(pyrazine-2- carboxamido)acetamido)-3,3-dimethylbutanoic acid (II)
A 1M aqueous solution of sodium hydroxide (440 ml, 440 mmol) is added to a solution of methyl (S)-2-((S)-2-cyclohexyl-2-(pyrazine-2- carboxamido)-3,3-dimethylbutanoate (28.7 g, 73.5 mmol) in methanol(150 ml) maintained under inert atmosphere, and the mixture is heated to 40-45 °C and maintained under stirring for 24 hours. The mixture is acidified with 37% hydrochloric acid to a pH of 1 -2 and extracted with ethyl acetate (3×200 ml); the combined organic phases are washed with a saturated solution of sodium chloride (200 ml), dried with sodium sulphate, and concentrated to residue. The crude product obtained is taken up with ethyl acetate, heated at reflux temperature for about an hour and cooled in 4-5 hours to 0-5°C, and the solid is filtered by washing with ethyl acetate (3×50 ml). The solid is dried at 45-50°C at low pressure and 26.5 g of crystalline product is obtained, with an HPLC purity of 99.7% and a yield of 95%. -NMR 300 MHz, δ (CD3OD): 9,23 (s, 1H); 8,77 (s, 1H); 8,66 (s, 1H); 8,30 (bd, J=9Hz, 1H); 4,65 (d, J=7Hz, 1H); 4,32-4,30 (m, 1H); 1 ,88-1 ,59 (m, 6H); 1 ,24-1 ,07 (m, 5H); 0,99 (s, 9H). 13C-NM 100 MHz, δ (CD3OD): 172,05; 171 ,45; 162,95; 147,06; 143,95; 143,08; 142,93; 60,38; 57,77; 39,91 ; 33,09; 28,07; 26,26; 25,91 ; 25,56; 25,21 ; 24,84. MS(ES+): m/z 399 [M+Na]+. | 95% |
![Route of Synthesis (ROS) of (S)-2-((S)-2-CYCLOHEXYL-2-[(PYRAZINE-2-CARBONYL)-AMINO]-ACETYLAMINO)-3,3-DIMETHYL-BUTYRIC ACID CAS 402958-96-7](https://www.chemwhat.com/wp-content/uploads/2017/12/Route-of-Synthesis-ROS-of-S-2-S-2-CYCLOHEXYL-2-PYRAZINE-2-CARBONYL-AMINO-ACETYLAMINO-33-DIMETHYL-BUTYRIC-ACID-CAS-402958-96-7.png)