Sulfamethoxazole EP Impurity A2 CAS#: 723-46-620022010; ChemWhat Code: 1409495
Identification
| Product Name | Sulfamethoxazole EP Impurity A2 |
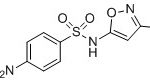
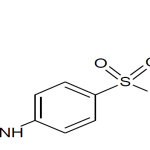
| IUPAC Name | 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide |
| Molecular Structure | |
| CAS Registry Number | 462-08-8 |
| EINECS Number | 207-322-2 |
| MDL Number | MFCD00006400 |
| Beilstein Registry Number | 105692 |
| Synonyms | sulfamethoxazole 723-46-6 Gantanol Sulphamethoxazole Sulfisomezole Sulfamethoxazol Metoxal 4-Amino-N-(5-methyl-3-isoxazolyl)benzenesulfonamide Sulfamethylisoxazole Simsinomin Radonil Sinomin Sulphamethoxazol 4-amino-N-(5-methylisoxazol-3-yl)benzenesulfonamide Sulpha-methoxizole Bactrim Sulfamethalazole Azo-gantanol Sulphamethylisoxazole Ro 4-2130 Urobak 3-Sulfanilamido-5-methylisoxazole Gantanol-DS 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide Sulfamethoxazolum Sulphisomezole Benzenesulfonamide, 4-amino-N-(5-methyl-3-isoxazolyl)- 5-Methyl-3-sulfanilamidoisoxazole MS 53 3-Sulphanilamido-5-methylisoxazole 5-Methyl-3-sulphanil-amidoisoxazole 3-(p-Aminophenylsulfonamido)-5-methylisoxazole 5-Methyl-3-sulfanylamidoisoxazole N’-(5-Methyl-3-isoxazolyl)sulfanilamide N1-(5-Methyl-3-isoxazolyl)sulfanilamide N’-(5-Methylisoxazol-3-yl)sulphanilamide 3-(para-Aminophenylsulphonamido)-5-methylisoxazole Sulmeprim SMX Trimeth/Sulfa A047 Sulfanilamide, N1-(5-methyl-3-isoxazolyl)- N(sup 1)-(5-Methyl-3-isoxazolyl)sulphanilamide CHEBI:9332 N’-(5-Methyl-3-isoxazole)sulfanilamide Sulfamethoxizole Ro 6-2580/11 STX-608 4-Amino-N-(5-methyl-isoxazol-3-yl)-benzenesulfonamide Sulfanilamide, N’-(5-methyl-3-isoxazolyl)- 4-AMINO-N-(5-METHYL-3-ISOXAZOYL)BENZENESULFONAMIDE Bactrimel Gamazole MFCD00010546 N(sup 1)-(5-Methyl-3-isoxazolyl)sulfanilamide CHEMBL443 NSC-147832 Ro-4-2130 MLS000069732 JE42381TNV Sulfametoxazol 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzene-1-sulfonamide Solfametossazolo N(sup1)-(5-Methyl-3-isoxazolyl)sulfanilamide 5-Methyl-3-sulfonylamidoisoxazole 3-(p-Aminobenzenesulfonamido)-5-methylisoxazole NSC147832 4-Amino-N-(5-methyl-3-isoxazolyl)-benzenesulfonamide 144930-01-8 NCGC00016533-05 NCGC00186654-01 CAS-723-46-6 SMR000058223 Sulfamethoxazole 100 microg/mL in Acetonitrile Solfametossazolo [DCIT] 3-METHYL-1-(4-SULFOAMIDOPHENYL)-5-PYRA& Sulfametoxazol [INN-Spanish] Sulfamethoxazolum [INN-Latin] Benzenesulfonamide, 4-amino-N-(5-methyl-3-isoxazolyl)-, radical ion(1+) CCRIS 567 HSDB 3186 SR-01000000217 EINECS 211-963-3 NSC 147832 BRN 0226453 UNII-JE42381TNV Sulfanilamide, N(1)-(5-methyl-3-isoxazolyl)- 08D Prestwick_453 ALBB-002089 Septran (Salt/Mix) Septrin (Salt/Mix) Sulfamethoxazole,(S) Eusaprim (Salt/Mix) Spectrum_000994 Sulfamethoxazole(USAN) starbld0000281 Opera_ID_882 Maybridge1_007190 Prestwick0_000177 Prestwick1_000177 Prestwick2_000177 Prestwick3_000177 Spectrum2_000788 Spectrum3_000584 Spectrum4_000345 Spectrum5_000982 Sulfamethoxazole [USAN:USP:INN:BAN:JAN] Epitope ID:114999 Co-trimoxazole (Salt/Mix) SCHEMBL3656 Oprea1_114486 Oprea1_285680 BSPBio_000073 BSPBio_002028 KBioGR_000749 KBioSS_001474 SULFAMETHOXAZOLE [MI] MLS001055354 MLS001074165 MLS006011871 BIDD:GT0731 DivK1c_000649 SPECTRUM1500550 SULFAMETHOXAZOLE [INN] SULFAMETHOXAZOLE [JAN] SPBio_000896 SPBio_001994 SULFAMETHOXAZOLE [HSDB] SULFAMETHOXAZOLE [IARC] SULFAMETHOXAZOLE [USAN] (4-aminophenyl)sulfonylamine BPBio1_000081 SULFAMETHOXAZOLE [VANDF] DTXSID8026064 SULFAMETHOXAZOLE [MART.] component of Bactrim (Salt/Mix) GTPL10933 HMS502A11 HMS561O18 JLKIGFTWXXRPMT-UHFFFAOYSA- KBio1_000649 KBio2_001474 KBio2_004042 KBio2_006610 KBio3_001528 ZINC89763 N1-(5-methylisoxazol-3-yl)-4-aminobenzene-1-sulfonamide STX 608 SULFAMETHOXAZOLE [USP-RS] SULFAMETHOXAZOLE [WHO-DD] SULFAMETHOXAZOLE [WHO-IP] WLN: T5NOJ C1 EMSWR DZ NINDS_000649 HMS1568D15 HMS1921A21 HMS2092K03 HMS2095D15 HMS2233L13 HMS3259E06 HMS3372M22 HMS3655O22 HMS3712D15 Pharmakon1600-01500550 BCP02881 HY-B0322 Sulfamethoxazole (JP17/USP/INN) Sulfamethoxazole, analytical standard Tox21_110480 Tox21_200353 BBL004554 BDBM50029770 CCG-40166 NSC757328 Ro-42130 s1915 STK007988 SULFAMETHOXAZOLE [ORANGE BOOK] AKOS000200952 component of Azo Gantanol (Salt/Mix) Tox21_110480_1 BS-3542 DB01015 NC00537 NSC-757328 RP-2145 SULFAMETHOXAZOLE [EP MONOGRAPH] COTRIM COMPONENT SULFAMETHOXAZOLE IDI1_000649 SEPTRA COMPONENT SULFAMETHOXAZOLE SULFAMETHOXAZOLE [USP MONOGRAPH] SULFAMETHOXAZOLUM [WHO-IP LATIN] BACTRIM COMPONENT SULFAMETHOXAZOLE NCGC00016533-01 NCGC00016533-02 NCGC00016533-03 NCGC00016533-04 NCGC00016533-06 NCGC00016533-07 NCGC00016533-08 NCGC00016533-09 NCGC00016533-10 NCGC00016533-11 NCGC00016533-12 NCGC00016533-14 NCGC00021995-03 NCGC00021995-04 NCGC00021995-05 NCGC00257907-01 UROPLUS COMPONENT SULFAMETHOXAZOLE AC-11118 SY018888 BCP0726000283 N’-(5-Methyl-3-isoxazolyl)-Sulfanilamide N1-(5-methyl-3-isoxazolyl)-Sulfanilamide N1-(5-Methyl-3-isoxazolyl)sulphanilamide SBI-0051524.P003 SULFAMETHOXAZOLE COMPONENT OF COTRIM SULFAMETHOXAZOLE COMPONENT OF SEPTRA SULFATRIM COMPONENT SULFAMETHOXAZOLE SULMEPRIM COMPONENT SULFAMETHOXAZOLE BACTRIM DS COMPONENT SULFAMETHOXAZOLE Co-trimethoxazole component sulfamethoxazole DB-055629 SULFAMETHOXAZOLE COMPONENT OF BACTRIM SULFAMETHOXAZOLE COMPONENT OF UROPLUS AB00052099 BB 0242379 FT-0602616 N^1-(5-Methyl-3-isoxazolyl)-Sulfanilamide SW196670-3 AZO GANTANOL COMPONENT SULFAMETHOXAZOLE COTRIM D.S. COMPONENT SULFAMETHOXAZOLE EN300-18400 SULFAMETHOXAZOLE COMPONENT OF SULFATRIM SULFAMETHOXAZOLE COMPONENT OF SULMEPRIM C07315 D00447 F12027 SULFAMETHOPRIM COMPONENT SULFAMETHOXAZOLE SULFAMETHOXAZOLE COMPONENT OF BACTRIM DS AB00052099-14 AB00052099-16 AB00052099_17 AB00052099_18 Sulfamethoxazole 1000 microg/mL in Acetonitrile SULFAMETHOXAZOLE COMPONENT OF AZO GANTANOL SULFAMETHOXAZOLE COMPONENT OF COTRIM D.S. BACTRIM PEDIATRIC COMPONENT SULFAMETHOXAZOLE Ndimethyl1-(5-methyl-3-isoxazolyl)-Sulfanilamide Q415843 SULFAMETHOXAZOLE COMPONENT OF SULFAMETHOPRIM Q-201762 SR-01000000217-2 SR-01000000217-3 Sulfamethoxazole, VETRANAL(TM), analytical standard 4-Amino-N-(5-methylisoxazol-3-yl)benzenesulphonamide BRD-K28494619-001-05-0 BRD-K28494619-001-15-9 BRD-K28494619-001-26-6 SULFAMETHOXAZOLE COMPONENT OF BACTRIM PEDIATRIC Z57198677 4-Amino-N-(5-methyl-isoxazol-3-yl)-benzene sulfonamide Sulfamethoxazole, British Pharmacopoeia (BP) Reference Standard Sulfamethoxazole, certified reference material, TraceCERT(R) 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide; SMX; SMZ Sulfamethoxazole, European Pharmacopoeia (EP) Reference Standard Sulfamethoxazole, United States Pharmacopeia (USP) Reference Standard Sulfamethoxazole, Pharmaceutical Secondary Standard; Certified Reference Material 129378-89-8 |
| Molecular Formula | C10H11N3O3S |
| Molecular Weight | 253.28 |
| InChI | InChI=1S/C10H11N3O3S/c1-7-6-10(12-16-7)13-17(14,15)9-4-2-8(11)3-5-9/h2-6H,11H2,1H3,(H,12,13) |
| InChI Key | JLKIGFTWXXRPMT-UHFFFAOYSA-N |
| Canonical SMILES | CC1=CC(=NO1)NS(=O)(=O)C2=CC=C(C=C2)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110117240 | Thiamine disulfide compound and synthesizing method and application thereof | 2019 |
| US2016/158251 | COMPOSITION FOR PREVENTING OR TREATING MUSCULAR ATROPHY OR PROMOTING MUSCULAR REGENERATION IN SUBJECT COMPRISING SULFONAMIDE COMPOUND AND USE THEREOF | 2016 |
| WO2005/42513 | PHENYL CARBOXAMIDE AND SULFONAMIDE DERIVATIVES FOR USE AS 11-BETA-HYDROXYSTEROID DEHYDROGENASE | 2005 |
Physical Data
| Appearance | A white or almost white, crystalline powder. |
| Melting Point, °C | Solvent (Melting Point) |
| 190 | |
| 167 | |
| 170.2 | |
| 168 – 172 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.493 | 19.84 | |
| 19.84 | ||
| 1.49 | 19.84 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | methanol | Boc-Leu-Aib-Phe-Phe-Aib-OMe | |
| Stability constant of the complex with … | H2O | 25 | β-cyclodextrin |
| Further physical properties of the complex | H2O | 25 | β-cyclodextrin |
| Stability constant of the complex with … | dioxane, H2O | 35 | copper |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Solid state NMR, Spectrum | 13C | |||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 26.84 | |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 26.84 |
| Description (IR Spectroscopy) |
| ATR (attenuated total reflectance), Spectrum |
| Bands, Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | ||||
| Spectrum | acetonitrile |
Route of Synthesis (ROS)

| Conditions | Yield |
| With hydrogenchloride In methanol; diethyl ether at 75℃; for 0.25h; Microwave irradiation; | 100% |
| With sodium hydroxide In water for 4h; Reflux; | 93% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H315 (55.56%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (56.48%): May cause an allergic skin reaction [Warning Sensitization, Skin] H319 (54.63%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (56.48%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H400 (34.26%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (34.26%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P313, P337+P317, P362+P364, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | No data available |
| Storage | Under room temperature away from light |
| Shelf Life | 5 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 253.282 |
| logP | 1.181 |
| HBA | 4 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 106.6 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Quantitative Results | ||
| 1 of 1,729 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Sulfur substituted sulfonylaminocarboxylic acid N-arylamides, their preparation, their use and pharmaceutical preparations comprising them | |
| 2 of 1,729 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Pharmaceutical vehicle | |
| 3 of 1,729 | Comment (Pharmacological Data) | Bioactivities present |
| Reference |
| Use Pattern |
| Sulfamethoxazole EP Impurity A2 CAS#: 723-46-620022010 is mainly used to treat acute and chronic urinary tract infections, and can also be used to prevent meningitis and acute otitis media caused by influenza bacilli. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to [email protected] |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |