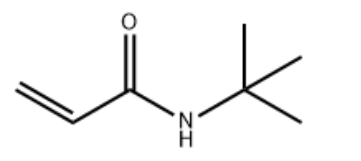
Tert-Butyl Acrylamide (n-TBAA) CAS#: 107-58-4; ChemWhat Code: 22973
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US10737259 | Salt tolerant anion exchange medium | 2020 |
| JP2017/186303 | MANUFACTURING METHOD OF β-SUBSTITUTED PROPIONIC ACID AMIDE AND N-SUBSTITUTED (METH)ACRYLAMIDE | 2017 |
| JP2015/209419 | METHOD OF PRODUCING N-SUBSTITUTED (METH)ACRYLAMIDE | 2015 |
| US2012/231072 | THERMO-RESPONSIVE HYDROGEL COMPOSITIONS | 2012 |
| US2008/286221 | Anionic Ethyl Methacrylate Copolymers and Use Thereof | 2008 |
Physical Data
| Appearance | White powder |
| Melting Point, °C | Solvent (Melting Point) |
| 130 – 131 | hexane |
| 124 – 125 | |
| 125 – 127 | |
| 123 – 124 | |
| 129 | |
| 128 – 131 | water |
| Density, g·cm-3 |
| 1 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the complex | 24 – 44 | N-Isopropylacrylamide, N,N-Dimethylacrylamide |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts, Spectrum | 13C | chloroform-d1 | ||
| Chemical shifts, Spectrum | 1H | CD3OD | ||
| Chemical shifts | 1H | chloroform-d1 | 500 | |
| Chemical shifts | 1H | chloroform-d1 | 24.84 | 600 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| ATR (attenuated total reflectance), Bands | |
| Bands, Spectrum | |
| Bands, Spectrum | potassium bromide |
| Bands | nujol |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| UV/VIS | ||
| Spectrum | ||
| Spectrum | 240 – 270 nm |
Route of Synthesis (ROS)
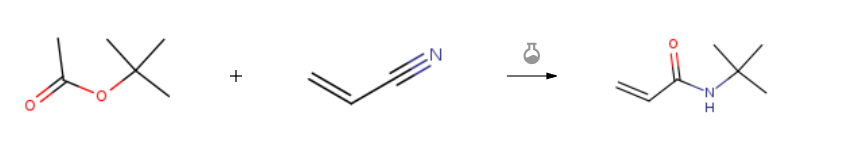
Route of Synthesis (ROS) of Tert-Butyl Acrylamide (n-TBAA) CAS 107-58-4
| Conditions | Yield |
| With BF3 immobilized on β-cyclodextrine functionalized silica coated CoFe2O4 magnetic nanoparticles In neat (no solvent) at 20℃; for 0.5h; Ritter Amidation; | 95% |
| With (2,3,4,5,6-pentafluorophenyl)ammonium triflate; water at 90℃; for 3h; Ritter reaction; Neat (no solvent); chemoselective reaction; | 92% |
| With sulfuric acid In acetic acid at 20℃; for 2.5h; Ritter reaction; Inert atmosphere; Enzymatic reaction; | 85% |
| Experimental Procedure General procedure: tert-butyl acetate (2 mmol), nitrile (2.2 mmol), and H2O (2 mmol) were mixed with PFPAT (10 mol %) and heated to 90 °C, until complete disappearance of the starting nitriles (as monitored by TLC). After cooling to room temperature, the organic phase was washed with aqueous 1 M NaOH solution (1 ml). The separated organic phase was evaporated under reduced pressure to give a crude residue, which was purified by distillation or by column chromatography (hexane-EtOAc). The products were characterized by comparison of their physical and spectral data with those of authentic samples. Spectroscopic data for selected examples: |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H319 (67.37%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P264, P264+P265, P270, P280, P301+P317, P305+P351+P338, P330, P337+P317, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| Under the room temperature and away from light | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 127.186 |
| logP | 1.302 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 29.1 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| Tert-Butyl Acrylamide (n-TBAA) CAS#: 107-58-4 is white powder. It is a monomer, is used for the production of many polymers and is an intermediate in organic chemical synthesis. (1) Industrial use (2) Paper industries (3) Personal care (4) Thickener. And N-tert-butylacrylamide is an important monomer that can be used in polymerization reactions to prepare high molecular weight polymers. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |