Tetrahydrothiophene CAS#: 110-01-0; ChemWhat Code: 17355
Identification
| Product Name | Tetrahydrothiophene |
| IUPAC Name | thiolane |
| Molecular Structure | 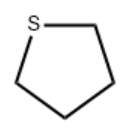 |
| CAS Registry Number | 110-01-0 |
| EINECS Number | 203-728-9 |
| MDL Number | MFCD00005476 |
| Beilstein Registry Number | 102392 |
| Synonyms | tetrahydrothiophene Thiolane 110-01-0 Thiophene, tetrahydro- Thiophane Thiacyclopentane Tetramethylene sulfide Thilane Tetrahydrothiophen Pennodorant 1013 Thiofan Thiolan Tetrahydrothiofen NSC 5272 744EHT13FM DTXSID3047760 CHEBI:48458 NSC-5272 Thiofan [Czech] Tetramethylenesulfide Pennodorant 1073 Tetrahydrothiofen [Czech] TETRAHYDROTHIOPHENE-2,2,5,5-D4 HSDB 6122 EINECS 203-728-9 UN2412 UNII-744EHT13FM AI3-30989 tetrahydro-thiophene MFCD00005476 Tetramethylene sulphide TRIMETHYLENESULFIDE Tetrahydrothiophene, 99% EC 203-728-9 CHEMBL1379 TETRAHYDROTHIOPHENE [MI] DTXCID8027743 NSC5272 Tox21_304026 AKOS006220479 Tetrahydrothiophene, analytical standard UN 2412 NCGC00357279-01 CAS-110-01-0 FT-0659266 T0114 EN300-105780 InChI=1/C4H8S/c1-2-4-5-3-1/h1-4H Q412118 Tetrahydrothiophene [UN2412] [Flammable liquid] W-108699 |
| Molecular Formula | C4H8S |
| Molecular Weight | 88.17 |
| InChI | InChI=1S/C4H8S/c1-2-4-5-3-1/h1-4H2 |
| InChI Key | RAOIDOHSFRTOEL-UHFFFAOYSA-N |
| Isomeric SMILES | C1CCSC1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN113999131 | Method for visible light to promote nickel-catalyzed alkyl C-H bonding to prepare amide derivatives | 2022 |
| JP2020/180120 | CARBOXYLATE, CARBOXYLIC ACID GENERATOR, RESIST COMPOSITION, AND METHOD FOR PRODUCING RESIST PATTERN | 2020 |
| CN112125891 | N2-selective tetrahydrofuran/tetrahydrothiophene substituted triazole derivative as well as synthesis method and application thereof | 2020 |
| CN109608462 | 7-alkyl-9-alkoxy/mercaptopurine-8-one compound, synthesis method thereof and application of compound in medicines | 2019 |
| WO2018/203194 | 2018 |
Physical Data
| Appearance | Colourless liquid |
| Boiling Point | 118~124°C |
| Melting Point, °C |
| -96.1 |
| -96.8 |
| -96.16 |
| -96.06 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 119 | |
| 119 | 760.051 |
| 121.117 | 760 |
| 122 – 124 | 760 |
| 119 – 121 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.99869 | 20 | |
| 1 | 4 | 20 |
| 0.9992 | 4 | 20 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Enthalpy of adsorption | hexane | 25 | zeolite Y |
| Further physical properties of the adsorbed molecule | -264.16 | Cu(001) | |
| Desorption | 25 – 200 | 7 percent MoO3/γ-Al2O3 surface | |
| Further physical properties of the adsorbed molecule | 24.9 | MoO3/γ-Al2O3 (initial) | |
| Enthalpy of adsorption |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | supporting information | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | supporting information | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | supporting information |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Spectrum | potassium bromide | |
| Bands | neat (no solvent) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | N,N-dimethyl-formamide, water | |||
| Spectrum | cyclohexane | 200 – 280 nm | ||
| Absorption maxima | cyclohexane | 202, 219 | 2480, 870 | |
| Spectrum | 180 – 230 nm |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Tetrahydrothiophene CAS 110-01-0
| Conditions | Yield |
| In ethanol; water at 20℃; for 2h; Experimental Procedure Tetrachloroauric(III) acid (1.0 g, 2.5 mmol) in 1.3 mL of water was added to 7.8 mL of ethanol andstirred at room temperature. To the resultant solution, 0.44 mL (4.9 mmol) of tetrahydrothiophene was addedslowly, stirred at room temperature for 2 h, and then white precipitate was appeared. The precipitate wascollected by filtration, washed with small amount of ethanol and air-dried to give 0.77 g (2.4 mmol) of whitesolid ((tht)AuCl) in 96% yield. | 96% |
| In ethanol; water at 20℃; for 0.25h; | 95% |
| In ethanol; water at 20℃;88%ethanol; water at 20℃; | 88% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H225: Highly Flammable liquid and vapor [Danger Flammable liquids] H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] H412: Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P317, P321, P330, P332+P317, P337+P317, P362+P364, P370+P378, P403+P235, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Keep container tightly closed in a dry and well-ventilated place. Keep away from heat andsources of ignition. |
| HS Code | |
| Storage | Keep container tightly closed in a dry and well-ventilated place. Keep away from heat andsources of ignition. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 88.1735 |
| logP | 1.687 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 25.3 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| Tetrahydrothiophene CAS#: 110-01-0 is used as an organic solvent, commonly employed in chemical synthesis and laboratory operations, particularly in reactions involving some sulfur compounds. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |