TETRAVINYLSILANE CAS#: 1112-55-6; ChemWhat Code: 66514
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2020/79219 | METHOD FOR PRODUCING TETRAALKENYLSILANE | 2020 |
| US2014/296468 | Hydrosilylation Reaction Catalysts and Curable Compositions and Methods for Their Preparation and Use | 2014 |
Physical Data
| Appearance | clear liquid |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 125 | |
| 130.6 | |
| 130.2 | 746.1 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.997 | ||
| 0.7984 | 4 | 20 |
| 0.7926 | 4 | 25 |
| 0.8 | 4 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | 400.1 | |
| Spectrum | 29Si | various solvent(s) | 59 |
| Chemical shifts | 13C | CDCl3 | |
| Chemical shifts | 13C | ||
| Chemical shifts | 29Si |
| Description (IR Spectroscopy) |
| Bands |
| Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
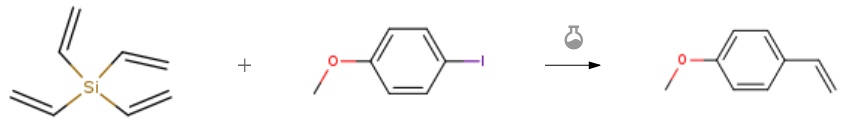
| With palladium(II) acetylacetonate; potassium fluoride In N,N-dimethyl-formamide at 130℃; under 1500.15 Torr; for 3h; Reagent/catalyst; Time; Hiyama Coupling; Inert atmosphere; Experimental Procedure General procedure: A mixture of organohalides (1a-h) (0.25mmol), vinysilanes 2a (0.3mmol) or 2c (0.075mmol), potassium fluoride (0.6mmol), and supported palladium NPs catalyst (Pd/substrate ratio 1mol%) was suspended in DMF (1ml). Then, the flask was evacuated under vacuum and refilled with argon. The evacuation/refilling cycle was repeated three times (pressure 2bar). The mixture was stirred at 130°C, and the reaction was monitored by GC and GC-MS. The reaction was performed for the time required ranging from 1.5 to 24h, to obtain the maximum yield of 3a-h products. | 93% |
Safety and Hazards
| Pictogram(s) | |
| Signal | Danger |
| GHS Hazard Statements | H225 (100%): Highly Flammable liquid and vapor [Danger Flammable liquids] H315 (74.51%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (74.51%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (19.61%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H361 (23.53%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H400 (23.53%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (23.53%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P203, P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P271, P273, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P317, P318, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P391, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 136.269 |
| logP | 3.544 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Tetraethoxysilane plays significant roles in semiconductor, photovoltaic, surface modification, and nanotechnology fields. Tetraethoxysilane can be employed in chemical vapor deposition (CVD) processes for the growth of silicon films, which are utilized in the semiconductor industry for integrated circuit fabrication. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |