TRIETHANOLAMINE BORATE CAS#: 283-56-7; ChemWhat Code: 57505
Identification
| Product Name | TRIETHANOLAMINE BORATE |
| IUPAC Name | 2,8,9-trioxa-5-aza-1-borabicyclo[3.3.3]undecane |
| Molecular Structure | 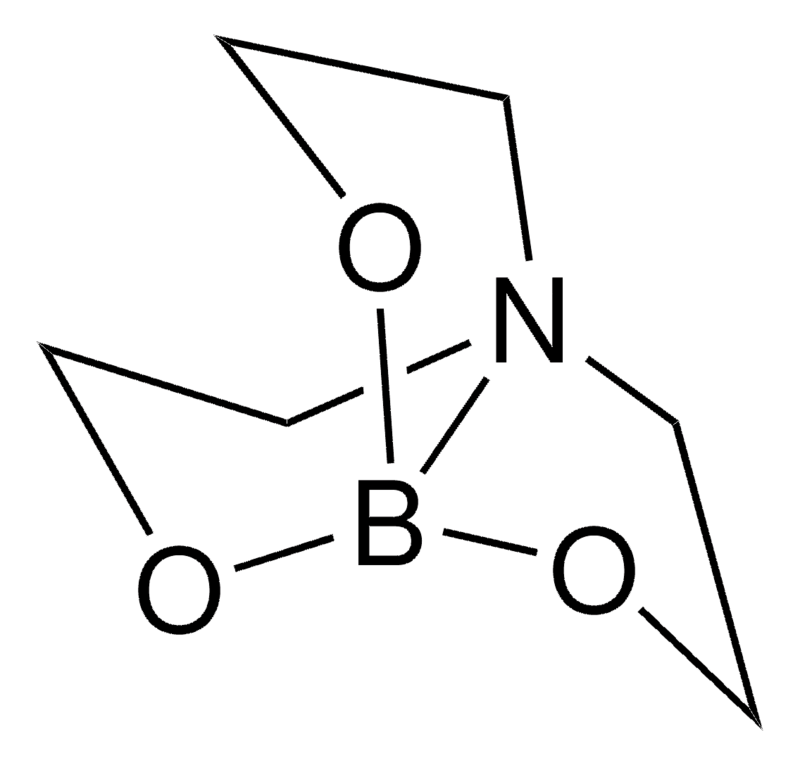 |
| CAS Registry Number | 283-56-7 |
| EINECS Number | 239-317-6 |
| MDL Number | MFCD00003272 |
| Beilstein Registry Number | 774536 |
| Synonyms | 2,8,9-trioxa-5-aza-1-borabicyclo[3.3.3]undecanetriethanolamine borateboratrane |
| Molecular Formula | C6H12BNO3 |
| Molecular Weight | 156.978 |
| InChI | InChI=1S/C6H12BNO3/c1-4-9-7-10-5-2-8(1)3-6-11-7/h1-6H2 |
| InChI Key | NKPKVNRBHXOADG-UHFFFAOYSA-N |
| Canonical SMILES | C1CN2CCOB(O1)OCC2 |
| Patent Information |
| No data available |
Physical Data
| Appearance | White powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 227 | |
| 242 | 3-methyl-butan-1-ol |
| 236 | acetonitrile |
| 236.5 – 237.5 | pyridine |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.423 | 4 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 23.04 | 500 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 24.04 | 125 |
| 2D-NMR | 11B | |||
| Spectrum | 11B | |||
| Spectrum | 1H | D2O | 30 – 90 | 399.78 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) | Signals, cm-1 |
| ATR (attenuated total reflectance), Bands | |||
| Bands | CHCl3 | 1363 | |
| Spectrum | nujol | 4000 – 400 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | H2O, H2SO4 | Ratio of solvents: 66percent | 258 | 5740 |
| Absorption maxima | H2O, NaOH | Ratio of solvents: 0.1N | 232, 290 | 8600, 3120 |
Route of Synthesis (ROS)
| Conditions | Yield |
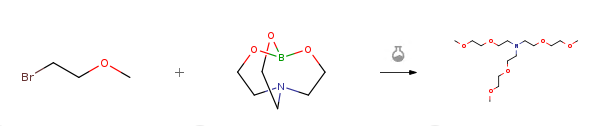
| With tetrabutyl-ammonium chloride; sodium hydroxide In sulfolane at 60 – 120℃; Reagent/catalyst; Experimental Procedure 2.1 139 g (1 mol) of 2-bromoethyl methyl ether was placed in the reaction flask.Triethanolamine borate 31.4g (0.2mol), tetrabutylammonium chloride 5.6g (0.02mol) and350 g of sulfolane, heated to 60 ° C,Start adding 32g (0.8mol) of sodium hydroxide in batches.After heating to 120 ° C for 3 to 4 hours, sampling GC detection,By rectification, 59.9 g of tris(3,6-dioxaheptyl)amine was obtained as a yellow oily liquid.Nuclear magnetic purity: 99.3%, yield: 92.7%. | 92.7% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 156.977 |
| logP | -0.411 |
| HBA | 4 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 30.93 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| The uses of triethanolamine borate (CAS No. 283-56-7) mainly rely on its structural characteristics, which is composed of triethanolamine (TEA) and boric acid or borate groups. The following are the main uses of this compound : |
| Main uses : Flame retardant : Due to the flame-suppressing properties of borates, triethanolamine borate is often used as a flame retardant in materials such as plastics and textiles to help improve the fire resistance of materials. |
| Corrosion inhibitor : Triethanolamine and borates are used as corrosion inhibitors in liquids such as metalworking fluids and coolants to effectively prevent oxidative corrosion of metals. |
| Lubricants and lubricant additives : As an additive in industrial lubricants such as lubricating oils and greases, triethanolamine borate helps to improve lubrication, reduce wear, and increase the stability and service life of lubricants. |
| Personal care and cosmetic formulations : Due to the good emulsification and pH adjustment capabilities of triethanolamine, triethanolamine borate may be used in cosmetics and personal care products such as creams, shampoos, skin care products, etc., acting as a pH regulator or emulsifier. |
| Water treatment and adhesives : Triethanolamine borate can also be used as a component of industrial chemicals such as water treatment agents, adhesives, sealants, etc., using its surface activity and buffering capacity to improve performance. |
| Summary : Triethanolamine borate is a versatile chemical that is mainly used in flame retardant, anti-corrosion, lubrication, cosmetic formulations and other fields, playing a role in adjusting pH, improving stability and protecting materials. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |