triethylindium CAS#: 923-34-2; ChemWhat Code: 1290789
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2014/78263 | METHODS OF PRODUCING TRIMETHYLGALLIUM | 2014 |
| US2004/122248 | Preparation of organometal compounds | 2004 |
| US2003/191333 | Trialkylindium preparation | 2003 |
Physical Data
No data available
| Melting Point, °C |
| -32 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.26 | 4 | 20 |
| Description (Association (MCS)) | Solvent (Association (MCS)) |
| Adsorption | CCl4 |
| Adsorption | indium antimonide |
| Adsorption | GaAs(100) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts, Spectrum | 1H | benzene-d6 | 25 |
| Chemical shifts, Spectrum | 1H | (2)H8-toluene | 25 |
| Description (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | 190 nm – 390 nm |
| Absorption maxima | Ratio of solvents: 0.1N |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of triethylindium CAS 923-34-2
| Conditions | Yield |
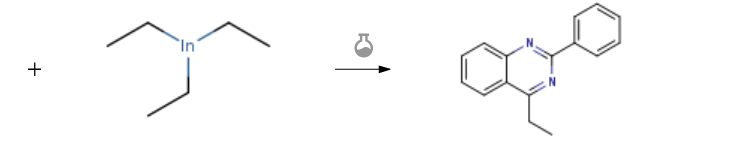
| With tris-(dibenzylideneacetone)dipalladium(0); tris(2-thienyl)phosphine In tetrahydrofuran at 60℃; for 4h; Inert atmosphere; Experimental Procedure General procedure: 4-Tosyloxyquinazoline derivatives 2 were obtained by using our reported method.5e A solution of quinazolin-4-ones 1 (0.2 mmol) in THF (2 mL) was treated with TsCl (1.2 equiv) and K2CO3 (3.0 equiv) at 60 °C. Upon completion of the reaction as indicated by TLC, the solvent was evaporated and the residue was purified on silica gel to give product 2. To a mixture of 4-tosyloxyquinazoline 2 (0.20 mmol) and organoindium reagent9 3 (1.0 equiv) in anhydrous THF (2.0 mL), Pd2(dba)3 (1 mol%) and (2-furyl)3P (2 mol%) were added. The mixture was stirred and heated at 60 °C under N2. Upon completion of the reaction as indicated by TLC, the solvent was evaporated. The residue was then diluted with EtOAc (10 mL), washed with H2O (10 mL), and dried with anhydrous MgSO4. Evaporation of the solvent followed by purification on silica gel (PE-EtOAc, 50:1 to 20:1) provided the corresponding product 4. | 73% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H250 (100%): Catches fire spontaneously if exposed to air [Danger Pyrophoric liquids] H314 (97.44%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] |
| Precautionary Statement Codes | P210, P222, P231, P233, P260, P264, P280, P301+P330+P331, P302+P335+P334, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 202.005 |
| logP | 2.658 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| triethylindium CAS#: 923-34-2 is a key precursor in thin film deposition technologies like CVD and ALD. It is used to deposit high-quality indium compound films in the semiconductor industry and other fields. Triethylindium can be employed in the preparation of indium compound films for use in optoelectronic devices, including solar cells and light-emitting diodes (LEDs). And Triethylindium is a crucial precursor in the semiconductor industry, used for depositing indium compound films, such as indium gallium alloys (InGaAs). These films have important applications in electronic devices and optoelectronics, such as high-electron-mobility transistors (HEMTs) and laser devices. Triethylindium is also utilized in metalorganic chemistry as an organometallic precursor, participating in some organic synthesis reactions. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |