Trimethoprim CAS#: 738-70-5; ChemWhat Code: 133633
Identification
| Product Name | Trimethoprim |
| IUPAC Name | 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine |
| Molecular Structure | 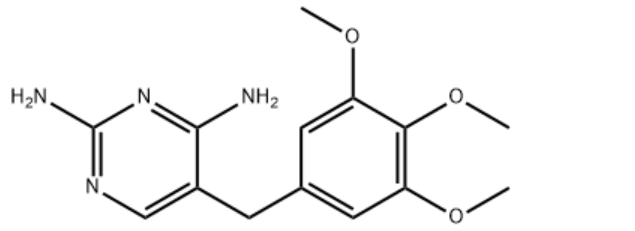 |
| CAS Registry Number | 738-70-5 |
| EINECS Number | 212-006-2 |
| MDL Number | MFCD00006400 |
| Beilstein Registry Number | 625127 |
| Synonyms | trimethoprim 738-70-5 Proloprim Trimpex Trimetoprim Bactramin Monotrim Monotrimin Trimopan Wellcoprim 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine Monoprim Syraprim Trimanyl Triprim Uretrim 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diamine Trimethoprimum Trimethoprime 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine NSC-106568 NIH 204 Primsol component of Bactrim BW 56-72 2,4-Pyrimidinediamine, 5-[(3,4,5-trimethoxyphenyl)methyl]- Infectotrimet TCMDC-125538 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine Trimethoprim-d9 5-[(3,4,5-Trimethoxyphenyl)methyl]-2,4-pyrimidinediamine NSC 106568 5-(3,4,5-Trimethoxybenzyl)-2,4-diaminopyrimidine BW-56-72 CHEBI:45924 CHEMBL22 Trimpex (TN) 2,4-Pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)methyl)- Abaprim MFCD00036761 Apo-Sulfatrim BW 5672 Pyrimidine, 2,4-diamino-5-(3,4,5-trimethoxybenzyl)- MLS000079023 AN164J8Y0X Briscotrim Novotrimel Streptoplus Sulfoxaprim Trimethioprim Urobactrim Wellcoprin Anitrim Antrima Antrimox Bacidal Bacticel Bactoprim Bencole Bethaprim Biosulten Chemotrin Colizole Conprim Cotrimel Duocide Esbesul Espectrin Euctrim Exbesul Fermagex Fortrim Ikaprim Kombinax Lagatrim Lastrim Metoprim Pancidim Protrin Resprim Salvatrim Setprin Sinotrim Sugaprim Sulfamar Sulthrim Sultrex Trimexol Trimezol Trimono Trisulcom Trisulfam Trisural Utetrin Velaten Xeroprim Zamboprim 2,4-Pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)-methyl)- Bacdan Bacide Deprim Omstat Purbal Roubac Roubal Stopan Toprim Trisul Bacin Bacta Futin NIH-204 Trimpex 200 Co-Trimoxizole Lagatrim Forte Septrin Forte Alcorim-F Colizole DS Septrin S Septrin DS Smz-Tmp NSC106568 Trimez-IFSA U-Prin component of Septra NCGC00016055-05 Trimethopriom 5-((3,4,5-Trimethoxyphenyl)methyl)-2,4-pyrimidinediamine Bactifor CAS-738-70-5 Dosulfin Instalac SMR000035999 Trimetoprim [DCIT] Trimogal Lescot Tiempe Trimetoprim [Polish] Resprim Forte Trimethoprim 100 microg/mL in Acetonitrile Uro-D S Tmp Smx 5-(3,4,5-Trimethoxy-benzyl)-pyrimidine-2,4-diamine Trimethoprime [INN-French] Trimethoprimum [INN-Latin] Trimetoprima [INN-Spanish] 2,4-Diamino-5-(3′,4′,5′-trimethoxybenzyl)pyrimidine Bacterial [Antibiotic] NIH 204 (VAN) Trimethoprim D3 (4-methoxy D3) Proloprim (TN) WR 5949 CCRIS 2410 HSDB 6781 SR-01000075652 EINECS 212-006-2 5-(3, 4, 5-Trimethoxybenzyl)-2, 4-pyrimidinediamine BRN 0625127 UNII-AN164J8Y0X Trimethoprim (JAN/USP/INN) 5-{[3,4,5-tris(methyloxy)phenyl]methyl}pyrimidine-2,4-diamine AI3-52594 Trimethoprim D3 (4-methoxy D3) 100 microg/mL in Acetonitrile B-Lock KUC103659N Trimethoprim,(S) Prestwick_485 KSC-4-158 Trimethoprim (TMP) Bactrim (Salt/Mix) Spectrum_000167 Tocris-0650 Trimethoprim [USAN:USP:INN:BAN:JAN] 2w9h 3fl9 3n0h 3s3v 4km2 Opera_ID_1760 Prestwick0_000208 Prestwick1_000208 Prestwick2_000208 Prestwick3_000208 Spectrum2_000937 Spectrum3_000643 Spectrum4_000372 Spectrum5_001559 Lopac-T-7883 TRIMETHOPRIM [MI] TRIMETHOPRIM [INN] TRIMETHOPRIM [JAN] Epitope ID:119684 UPCMLD-DP132 T 7883 TRIMETHOPRIM [HSDB] TRIMETHOPRIM [USAN] TRIMETHOPRIM [VANDF] Lopac0_001271 Oprea1_495058 SCHEMBL24506 BSPBio_000195 BSPBio_002245 KBioGR_000863 KBioSS_000647 TRIMETHOPRIM [MART.] 5-25-13-00429 (Beilstein Handbook Reference) MLS001201740 MLS002303068 MLS002548881 BIDD:GT0190 DivK1c_000589 SPECTRUM1500595 TRIMETHOPRIM [USP-RS] TRIMETHOPRIM [WHO-DD] SPBio_000874 SPBio_002116 BPBio1_000215 DTXSID3023712 UPCMLD-DP132:001 BDBM18069 GTPL10931 HMS501N11 KBio1_000589 KBio2_000647 KBio2_003215 KBio2_005783 KBio3_001465 TRIMETHOPRIM [GREEN BOOK] Trimethoprim, >=98% (HPLC) NINDS_000589 2,4,5-trimethoxybenzyl)pyrimidine HMS1568J17 HMS1921I03 HMS2090D14 HMS2092A10 HMS2095J17 HMS2230L06 HMS3259I11 HMS3263P04 HMS3371O18 HMS3652E03 HMS3712J17 Pharmakon1600-01500595 TRIMETHOPRIM [EP IMPURITY] TRIMETHOPRIM [ORANGE BOOK] Trimethoprim for system suitability TRIMETHOPRIM [EP MONOGRAPH] 2,4,5-trimethoxyphenzyl)pyrimidine ALBB-028968 BCP12148 COTRIM COMPONENT TRIMETHOPRIM HY-B0510 SEPTRA COMPONENT TRIMETHOPRIM ZINC6627681 Co-trimoxazole component trimethoprim Tox21_110291 Tox21_200157 Tox21_501271 TRIMETHOPRIM [USP MONOGRAPH] BACTRIM COMPONENT TRIMETHOPRIM BBL005584 CCG-40335 NSC752719 NSC757370 s3129 STK177322 STL455117 UROPLUS COMPONENT TRIMETHOPRIM TRIMETHOPRIMUM [WHO-IP LATIN] AKOS001650069 SULFATRIM COMPONENT TRIMETHOPRIM SULMEPRIM COMPONENT TRIMETHOPRIM Tox21_110291_1 AC-8427 BW-5672 DB00440 KS-1145 LP01271 NC00483 NSC-752719 NSC-757370 SDCCGSBI-0051237.P004 TRIMETHOPRIM COMPONENT OF COTRIM TRIMETHOPRIM COMPONENT OF SEPTRA BACTRIM DS COMPONENT TRIMETHOPRIM IDI1_000589 SMP2_000262 TRIMETHOPRIM COMPONENT OF BACTRIM TRIMETHOPRIM COMPONENT OF UROPLUS NCGC00016055-01 NCGC00016055-02 NCGC00016055-03 NCGC00016055-04 NCGC00016055-06 NCGC00016055-07 NCGC00016055-08 NCGC00016055-09 NCGC00016055-10 NCGC00016055-11 NCGC00016055-12 NCGC00016055-13 NCGC00016055-14 NCGC00016055-16 NCGC00016055-17 NCGC00016055-27 NCGC00024707-01 NCGC00024707-03 NCGC00024707-04 NCGC00024707-05 NCGC00024707-06 NCGC00024707-07 NCGC00024707-08 NCGC00257711-01 NCGC00261956-01 COTRIM D.S. COMPONENT TRIMETHOPRIM SY031734 TRIMETHOPRIM COMPONENT OF SULFATRIM TRIMETHOPRIM COMPONENT OF SULMEPRIM Trimethoprim/sulfamethoxazole (commercial) SBI-0051237.P003 DB-055812 SULFAMETHOPRIM COMPONENT TRIMETHOPRIM TRIMETHOPRIM COMPONENT OF BACTRIM DS 2, 5-[(3,4,5-trimethoxyphenyl)methyl]- AB00052118 BB 0258034 EU-0101271 FT-0601630 FT-0675578 FT-0675579 FT-0675580 SW196690-3 T2286 Trimethoprim 1000 microg/mL in Acetonitrile TRIMETHOPRIM COMPONENT OF COTRIM D.S. BACTRIM PEDIATRIC COMPONENT TRIMETHOPRIM C01965 D00145 EN300-118703 TRIMETHOPRIM COMPONENT OF SULFAMETHOPRIM Trimethoprim, crystallized, >=99.0% (HPLC) WLN: T6N CNJ BZ DZ E1R CO1 DO1 EO1 5-(3,5-Trimethoxybenzyl)-2,4-diaminopyrimidine AB00052118-30 AB00052118-32 AB00052118_33 AB00052118_34 Trimethoprim, VETRANAL(TM), analytical standard 738T705 Q422665 TRIMETHOPRIM COMPONENT OF BACTRIM PEDIATRIC 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-pyrimidine Pyrimidine,4-diamino-5-(3,4,5-trimethoxybenzyl)- SR-01000075652-1 SR-01000075652-3 SR-01000075652-6 W-104441 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine # BRD-K07208025-001-06-5 SR-01000075652-10 2-amino-5-(3,4,5-trimethoxybenzyl)-4-pyrimidinylamine F0914-5266 Trimethoprim, certified reference material, TraceCERT(R) Z1515385071 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4(1H,3H)-diimine Trimethoprim, British Pharmacopoeia (BP) Reference Standard Trimethoprim, European Pharmacopoeia (EP) Reference Standard Trimethoprim, United States Pharmacopeia (USP) Reference Standard Trimethoprim for system suitability, European Pharmacopoeia (EP) Reference Standard Trimethoprim, Pharmaceutical Secondary Standard; Certified Reference Material |
| Molecular Formula | C14H18N4O3 |
| Molecular Weight | 290.32 |
| InChI | InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) |
| InChI Key | IEDVJHCEMCRBQM-UHFFFAOYSA-N |
| Canonical SMILES | COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2017/29202 | HYPERPHENYLALANINEMIA AND TREATMENTS THEREOF | 2017 |
| US3985745 | 3-Imino-1,2,4-benzotriazine-1-oxides | 1976 |
| US4115650 | Process for preparing 2,4-diamino-5-(substituted benzyl)-pyrimidines | 1978 |
Physical Data
| Appearance | White Specifications or yellowish white powder |
| Melting Point, °C |
| 199 – 203 |
| 201.8 |
| 138 – 139 |
| 200 – 203 |
| Density, g·cm-3 | Reference Temperature, °C |
| 1.329 | -173.16 |
| 1.2 | 4 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | 17.84 | bovine serum albumin | |
| Association with compound | 17.84 | human serum albumin | |
| Association with compound | randomly methylated β-cyclodextrin | ||
| Association with compound | ethanol, H2O | 24.85 – 44.85 | montmorillonite K10 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | water-d2 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 300 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | ||
| ATR (attenuated total reflectance), Spectrum | ||
| Bands | potassium bromide |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | water | ||
| Spectrum | dichloromethane | Ratio of solvents: 0.1N | |
| Spectrum | aq. phosphate buffer | 270.6 |
Route of Synthesis (ROS)

| Conditions | Yield |
| With tin(IV) chloride In chloroform at 20℃ | 90.58% |
| With tin(IV) chloride In chloroform for 1h; Solvent; Reflux | 90.58% |
| With tin(IV) chloride In chloroform for 1h; Reflux | 90.58% |
| Experimental Procedure 8 Example 8 Preparation of compound 2 (N, N ‘-(5- (2-acetyl-3,4,5-trimethoxybenzyl) pyrimidine-2,4-diyl) diacetamide) Add Compound 1 (trimethoprim) (10.00g, 34.45mmol) to a 250ml reaction flask,Acetyl chloride (9.80 ml, 138.57 mmol) and 100 ml of chloroform, and tin tetrachloride (8.00 ml, 68.36 mmol) was added under stirring, and the reaction was refluxed for 1 h.Cool down to room temperature, pour the reaction solution into 50ml ice water, stir for 6mins, separate the liquid, wash the organic phase 3 times with 5ml water,Combine the aqueous phases, extract the aqueous phase 3 times with 5 ml of chloroform, combine the organic phases, adjust the pH of the saturated sodium carbonate aqueous solution to 7-8, and separate the liquid organic phase 5 times with the aqueous phase.It was dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The ethylene glycol monomethyl ether was recrystallized to obtain 12.98 g of the product with a yield of 90.58%. The purity was 95.52% by HPLC.erimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | 97% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H301: Toxic if swallowed [Danger Acute toxicity, oral] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] H362: May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H373: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H401: Toxic to aquatic life [Hazardous to the aquatic environment, acute hazard] H411: Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P203, P260, P263, P264, P270, P273, P280, P301+P316, P318, P319, P321, P330, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 5 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 290.322 |
| logP | 0.783 |
| HBA | 4 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 105.51 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Trimethoprim CAS#: 738-70-5 is a lipophilic weakly basic pyrimethamine bacteriostatic agent. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

