Triphenylsulfonium, 2-hydroxybenzoate (1:1) CAS#: 345580-99-6; ChemWhat Code: 1491753
Identification
| Product Name | Triphenylsulfonium, 2-hydroxybenzoate (1:1) |
| IUPAC Name | 2-carboxyphenolate;triphenylsulfanium |
| Molecular Structure | 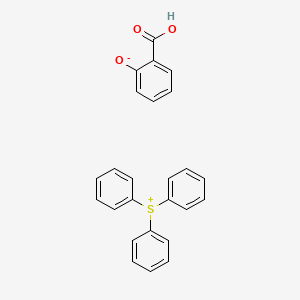 |
| CAS Registry Number | 345580-99-6 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | triphenylsulfonium 2-hydroxybenzoatetriphenylsulfonium salicylate |
| Molecular Formula | C25H20O3S |
| Molecular Weight | 400.5 |
| InChI | InChI=1S/C18H15S.C7H6O3/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;8-6-4-2-1-3-5(6)7(9)10/h1-15H;1-4,8H,(H,9,10)/q+1;/p-1 |
| InChI Key | KKLIEUWPBXKNFS-UHFFFAOYSA-M |
| Canonical SMILES | C1=CC=C(C=C1)[S+](C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C(=C1)C(=O)O)[O-] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| TW2025/28287 | Resin composition, film, pattern formation method, and electronic device manufacturing method | 2025 |
| KR2024/76711 | CARBOXYLATE, CARBOXYLIC ACID GENERATOR, RESIST COMPOSITION AND METHOD FOR PRODUCING RESIST PATTERN | 2024 |
Physical Data
| Appearance | White to off-white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
Spectra
| No data available |
Route of Synthesis (ROS)
| Conditions | Yield |
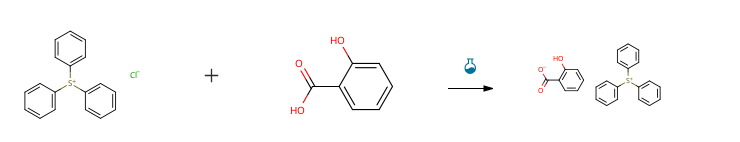
| With sodium hydrogencarbonate In water at 20℃; for 1.16667h; Experimental Procedure Synthesis of Sulfonium Salt D-1 Pure water was added to 10.0 g (72.4 mmol) of salicylic acid and 6.1 g (72.4 mmol) of sodium bicarbonate, and the mixture was stirred for 10 minutes. Then, 21.6 g (72.4 mmol) of triphenylsulfonium chloride was added. After stirring at room temperature for 1 hour, the layers were separated, and the resulting organic layer was washed with pure water. The solvent was then removed using a rotary evaporator to obtain a crude product. The resulting crude product was purified by silica gel chromatography to obtain 27.2 g (94% yield) of the desired product (sulfonium salt D-1). | 94% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H301 (88.9%): Toxic if swallowed [Danger Acute toxicity, oral] H315 (11.1%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (11.1%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P264+P265, P270, P280, P301+P316, P302+P352, P305+P351+P338, P321, P330, P332+P317, P337+P317, P362+P364, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 400.498 |
| logP | 6.693 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 60.36 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| I. Core Function: Photoacid Generator (PAG) This compound is a triphenylsulfonium salt-based photoacid generator (PAG). Under UV or deep-UV (DUV) irradiation: It absorbs light and undergoes photolytic cleavage Generates a strong acid The generated acid can catalyze chemically amplified reactions or initiate subsequent cationic polymerization. It is a key functional additive in photoresist and cationic photopolymer curing systems. II. Main Application Areas 1. Semiconductor Photoresists (Chemically Amplified Photoresists, CARs) Applicable to: i-line, KrF, and ArF lithography systems High-resolution chemically amplified photoresists Main functions: Acid generation upon exposure Catalyzes deprotection reactions Amplifies exposure effects → improves resolution, sensitivity, and critical dimension / line edge roughness (CD/LER) control Advantages of the ortho-hydroxybenzoate structure: Provides good photosensitivity Reduces acid diffusion, improving resolution Works synergistically with the triphenylsulfonium cation → good thermal stability and stable photoresist performance 2. Cationic Photocuring Systems Used in UV-induced cationic curing of epoxy resins and vinyl ether systems UV irradiation → acid generation → initiates cationic ring-opening polymerization or crosslinking Applications include: UV-curable coatings and inks Electronic encapsulation materials Photo-patternable insulating layers (e.g., PSPI, PI) |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| WatsonChem Advanced Chemical Materials | https://www.watsonchem.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |