Tropifexor CAS#: 1383816-29-2; ChemWhat Code: 1097529
Identification
| Product Name | Tropifexor |
| IUPAC Name | 2-[(1R,5S)-3-[[5-cyclopropyl-3-[2-(trifluoromethoxy)phenyl]-1,2-oxazol-4-yl]methoxy]-8-azabicyclo[3.2.1]octan-8-yl]-4-fluoro-1,3-benzothiazole-6-carboxylic acid |
| Molecular Structure | 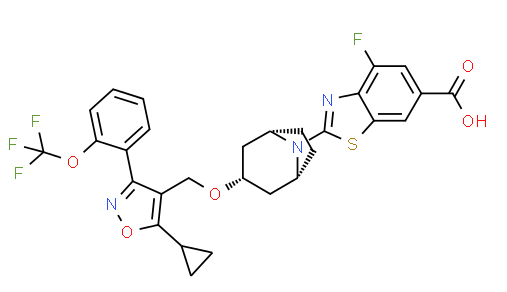 |
| CAS Registry Number | 1383816-29-2 |
| EINECS Number | No data available |
| MDL Number | MFCD31693060 |
| Beilstein Registry Number | No data available |
| Synonyms | 2-[(1R,3r,5S)-3-({5-cyclopropyl-3-[2-(trifluoromethoxy)phenyl]-1,2-oxazol-4-yl}methoxy)-8-azabicyclo[3.2.1]octan-8-yl]-4-fluoro-1,3-benzothiazole-6-carboxylic acid, 2-[3-({5-cyclopropyl-3-[2-(trifluoromethoxy)phenyl]1,2-oxazol-4-yl}methoxy)-8-azabicyclo[3.2.1]octan-8-yl]4-fluoro-1,3-benzothiazole-6-carboxylic acid, 2-(3-((5-cyclopropyl-3-(2-(trifluoromethoxy)phenyl)isoxazol-4-yl)methoxy)-8-azabicyclo[3.2.1]octan-8-yl)-4-fluorobenzo[d]thiazole-6-carboxylic acid, tropifexor, LJN452 |
| Molecular Formula | C29H25F4N3O5S |
| Molecular Weight | 603.584 |
| InChI | InChI=1S/C29H25F4N3O5S/c30-21-9-15(27(37)38)10-23-25(21)34-28(42-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-41-26(20)14-5-6-14)19-3-1-2-4-22(19)40-29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38)/t16-,17+,18+ |
| InChI Key | VYLOOGHLKSNNEK-PIIMJCKOSA-N |
| Canonical SMILES | c1ccc(c(c1)c2c(c(on2)C3CC3)CO[C@H]4C[C@H]5CC[C@@H](C4)N5c6nc7c(cc(cc7s6)C(=O)O)F)OC(F)(F)F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2012/87519 | COMPOSITIONS AND METHODS FOR MODULATING FXR | 2012 |
Physical Data
| Appearance | Powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 221 | acetonitrile |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| -173.16 | 4 | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 100 |
| Chemical shifts | 19F | dimethylsulfoxide-d6 | 376 |
| 1H | deuteromethanol | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | 27 |
| Spectrum | CCl4 | 14.85 – 54.85 |
| Description (UV/VIS Spectroscopy) |
| Bands |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), time-of-flight mass spectra (TOFMS), electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
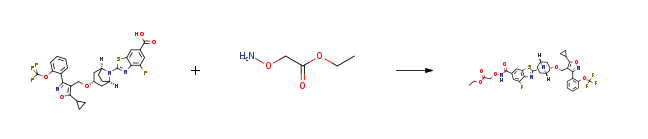
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; Inert atmosphere; Experimental Procedure 6.1 first step2-((2-((1R, 3R, 5S) -3-((5-cyclopropyl-3- (2- (trifluoromethoxy) phenyl) isoxazol-4-yl) methoxy ) -8-azabicyclo [3.2.1] oct-8-yl) -4-fluorobenzo [d] thiazole-6-formylamino) oxy) ethyl acetate Under the protection of argon, 2-((1R, 3R, 5S) -3-((5-cyclopropyl-3- (2- (trifluoromethoxy) phenyl) isoxazol-4-yl) (Methoxy) -8-azabicyclo [3.2.1] oct-8-yl) -4-fluorobenzo [d] thiazole-6-carboxylic acid 5a (180 mg, 0.3 mmol, according to published patent application “WO 2012087519 “, 2- (aminooxy) ethyl acetate 6a (53.6 mg, 0.45 mmol, prepared according to published patent application” WO 2002103008 “), 2- (7-azobenzotriazole) -N, N, N ‘, N’-Tetramethylurea hexafluorophosphate (HATU) (171 mg, 0.45 mmol) and triethylamine (60.6 mg, 0.2 mmol) were dissolved in 10 mL of N, N-dimethylformamide At room temperature, the reaction was allowed to proceed overnight. 100 mL of water was added, extraction was performed with ethyl acetate (100 mL), and the organic phase was washed with a saturated sodium chloride solution (100 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The obtained residue was subjected to thin layer chromatography (developed Agent: System A) was purified to obtain 2-((2-((1R, 3R, 5S) -3-((5-cyclopropyl-3- (2- (trifluoromethoxy) phenyl) isoxamine Azole-4-yl) methoxy) -8-azabicyclo [3.2.1] oct-8-yl) -4-fluorobenzo [d] thiazole-6-formylamino) oxy) ethyl acetate 6b (140 mg, yellow oil) | 66% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 603.594 |
| logP | 6.823 |
| HBA | 5 |
| HBD | 1 |
| Matching Lipinski Rules | 2 |
| Veber rules component | |
| Polar Surface Area (PSA) | 126.16 |
| Rotatable Bond (RotB) | 9 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Dose |
| 10 | mRNA expression level increase(of mRNA level) | Active | 0.0001 μM | ||
| 9.72 | EC50 | 0.00019 | µM | ||
| 9.7 | EC50 | 0.2 | nM | ||
| 9.59 | EC50 | 0.26 | nM | ||
| 9 | mRNA expression level increase(of mRNA level) | Active | 0.001 -1 μM | ||
| 1 | EC50 | > | 10 | µM | |
| 1 | EC50 | > | 10 | µM | |
| mRNA expression level increase(of mRNA level) | 3 | fold | 1 nM | ||
| mRNA expression level increase(of mRNA level) | > | 15 | fold | 10nM |
| In vivo: Animal Model |
| Quantitative Results |
| pX | Parameter | Value (quant) | Biological Species | Route of administration | Dose |
| 9.92 | concentration (parameter) | 0.12 | rat | oral administration | 0.003mg/kg |
| 9.7 | concentration (parameter) | 0.2 | rat | oral administration | 0.003mg/kg |
| 9.51 | concentration (parameter) | 0.31 | rat | oral administration | 0.01mg/kg |
| 9.43 | concentration (parameter) | 0.37 | rat | oral administration | 0.01mg/kg |
| 8.89 | concentration (parameter) | 1.16 | rat | oral administration | 0.03mg/kg |
| 8.64 | concentration (parameter) | 1.29 | rat | oral administration | 0.03mg/kg |
| 7.79 | concentration (parameter) | 16.41 | rat | oral administration | 1 mg/kg |
| 7.2 | concentration (parameter) | 62.61 | rat | oral administration | 3 mg/kg |
| Metabolism |
| Quantitative Results |
| Parameter | Value (qual) | Value (quant) | Unit |
| extraction ratio | 0.55 | no unit | |
| extraction ratio | < | 0.18 | no unit |
| Pharmacokinetic |
| Quantitative Results |
| Parameter | Value (quant) | Biological Species | Route of administration | Dose |
| CL (drug clearance) | 5.3 | mouse | intravenous administration | 5 mg/kg |
| Vdss | 0.7 | mouse | intravenous administration | 5 mg/kg |
| half life time | 2.6 | mouse | intravenous administration | 5 mg/kg |
| AUC | 4785 | mouse | oral administration | 10 mg/kg |
| Cmax | 1186 | mouse | oral administration | 10 mg/kg |
| plasma protein-bound fraction | 99 | dog | oral administration | 2 mg/kg |
| AUC (0-24 h) | 117 | rat | oral administration | 0.3 mg/kg |
| F (drug bioavailability) | 10 | rat | oral administration | 1 mg/kg |
| Use Pattern |
| Tropifexor CAS#: 1383816-29-2 as Pharmaceuticals |
| reducing at least one point in severity of nonalcoholic fatty liver disease grading scoring systems in combination with (S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-N-(tetrahydrothran-3-yl)pyrimidine-5-carboxamide and 4-(4-(1-isopropyl-7-oxo-1,4,6,7-tetrahydrospiro[indazole-5,4’-piperidine]-1’-carbonyl)-6-methoxypyridin-2-yl)benzoic acid |
| reducing at least one point in severity of nonalcoholic steatohepatitis grading scoring systems |
| FXR mediated disorder or condition |
| alcohol-induced cirrhosis |
| cholestasis of pregnancy |
| cystic fibrosis or liver fibrosis |
| Tropifexor CAS#: 1383816-29-2 used as for liver cirrhosis |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |