Vinyl acetate CAS#: 108-05-4; ChemWhat Code: 28034
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117466734 | Method for preparing lipoic acid intermediate by using continuous flow | 2024 |
| US2023/30626 | HYDROSILYLABLE MIXTURE INCLUDING STRONG ELECTRON DONORS, AS CATALYST ADDITIVE | 2023 |
| WO2023/21080 | CHEMICAL PROCESS FOR VINYL ETHER SYNTHESIS | 2023 |
| WO2023/201154 | SILICONE – VINYLESTER FUNCTIONAL COMPOUNDS AND METHODS FOR THEIR PREPARATION AND USE IN PERSONAL CARE COMPOSITIONS | 2023 |
| CN114805182 | Method for synthesizing indole spiro-propane compound through visible light catalysis | 2022 |
| CN114763339 | Preparation method of triazine derivative | 2022 |
Physical Data
| Appearance | Transparent liquid |
| Melting Point, °C | |
| -93 | |
| 72 | |
| -93.2 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 72.3 | |
| 72 – 73 | |
| 72 | |
| 73 | |
| 72.79 | 759.826 |
| 72 | |
| 72.95 | 759.811 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 0.90652 | 39.99 |
| 0.91307 | 34.99 |
| 0.91956 | 29.99 |
| 0.92601 | 24.99 |
| 0.93241 | 19.99 |
| 0.9128 | 34.99 |
| 0.9193 | 29.99 |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Desorption | manganese(IV) oxide | |
| Desorption | manganese dioxide | |
| Desorption | manganese dioxide | |
| Further physical properties of the adsorbed molecule | Pd(111)-(2*2)O surface | |
| Desorption | 109.9 – 149.9 | AR-3 charcoal |
| Adsorption |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Spectrum | 1H | chloroform-d1 | 24.84 |
| Spectrum | 1H | CD3OD | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Spectrum | 1H | ||
| Spectrum | 1H | dimethylsulfoxide-d6 | |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | ||
| ATR (attenuated total reflectance), Bands, Spectrum | ||
| Intensity of IR bands, Bands, Spectrum | potassium bromide | |
| Bands, Spectrum | potassium bromide | |
| Bands, Spectrum | methanol | 20.84 |
| Bands, Spectrum | propan-1-ol | 20.84 |
| Bands, Spectrum | ethanol | 20.84 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | ||
| Spectrum | ethanol | 210 – 270 nm |
| Spectrum | 250 – 360 nm, unverduennt. | |
| Spectrum | hexane | |
| Spectrum | der reinen Substanz. | |
| Spectrum | 2,2,4-trimethyl-pentane |
Route of Synthesis (ROS)
| Conditions | Yield |
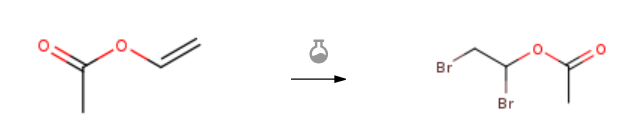
| With bromine In tetrachloromethane | 100% |
| With propane 3-bromo-1-(triphenylphosphonium) tribromide In dichloromethane at 20℃; for 0.5h; Experimental Procedure 3.3. General experimental procedure for bromination of alkenes General procedure: In a typical reaction, the alkene (3 mmol) was dissolved indichloromethane (5 mL) and stirred well for 2 min. Propane 3-bromo-1-(triphenyl phosphonium) tribromide II (3 mmol) dissolvedin dichloromethane (5 mL) andwas added to alkene solutiondropwise with constant stirring at room temperature. The progressof the reaction was monitored by TLC and GC. After completion ofthe reaction and disappearance of the yellow-orange color of reagentII, the solvent evaporated and diethyl ether was added(3 5 ml). The mixture was filtered and the solvent evaporated. The crude product thus obtained and then subjected to a shortcolumn of silica gel using a mixture of n-hexane and ethyl acetate(8:2) as the eluent. All of the isolated products are known andphysical data have been reported in the literature. The mainproducts, reaction times and isolated yields are tabulated in Table 2.To confirm, the products were subjected to DSC and GC/MS andspectra were compared and validated with the NIST library (seesupporting information). | 90% |
| With bromine for 3h; | 62% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H225 (> 99.9%): Highly Flammable liquid and vapor [Danger Flammable liquids] H332 (56.8%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (56.7%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H351 (57.8%): Suspected of causing cancer [Warning Carcinogenicity] H412 (28.9%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P203, P210, P233, P240, P241, P242, P243, P261, P271, P273, P280, P303+P361+P353, P304+P340, P317, P318, P319, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 86.0904 |
| logP | 0.694 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Vinyl acetate is the main raw material for the manufacture of synthetic fiber vinylon. Vinyl acetate is polymerized by itself or copolymerized with sub-monomers to obtain polyvinyl alcohol, vinyl acetate-ethylene copolymer (EVA), vinyl acetate-vinyl chloride copolymer (EVC), vinyl acetate-acrylonitrile fiber, and vinyl acetate-acrylic acid ester copolymer. They all have important industrial uses and are widely used as adhesives, architectural coatings, textile sizing agents and finishing agents, paper reinforcement agents, and for the manufacture of safety glass, etc. Vinyl acetate reacts with ethanol and bromine to produce bromoacetaldehyde diethyl acetal. This is an intermediate for the drug methimazole. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | https://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |