1-(2-NAPHTHYL)METHANAMINE CAS#: 2018-90-8; ChemWhat Code: 201624
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN114805106 | Preparation method of amide compound | 2022 |
| CN114773252 | Chiral amino indoline derivative as well as preparation method and application thereof | 2022 |
| CN106928037 | Preparation method of carvone | 2017 |
| CN107417623 | One-step synthesis method of 5-diarylamino benzimidazole derivatives | 2017 |
| EP2810944 | HETEROCYCLIC COMPOUND HAVING ANTI-HIV ACTIVITY | 2014 |
| US2007/179115 | Purinenucleoside derivative modified in 8-position and medical use thereof | 2007 |
| US6337398 | Succinoylamino hydroxyethylamino sulfonyl urea derivatives useful as retroviral protease inhibitors | 2002 |
Physical Data
| Appearance | White powder |
| Melting Point, °C |
| 55 – 57 |
| 55 – 56 |
| 55 – 56 |
| 269 |
| 58 |
| 59 – 60 |
| 60 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 143 – 147 | 8 |
| 180 | 24 |
| 148 – 149 | 12 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400.1 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100.6 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| ATR (attenuated total reflectance), Bands | neat (no solvent, solid phase) |
| ATR (attenuated total reflectance), Bands | |
| FT-IR, in KBr | |
| Bands | KBr |
| Bands | KBr |
Route of Synthesis (ROS)
| Conditions | Yield |
| With piperidin-2-one; sodium tetrahydroborate; N,N-diisopropyl-4H-benzo[d][1,3,2]dioxaborinin-2-amine; ammonia In 1,2-dichloro-ethane at 20℃; | 99% |
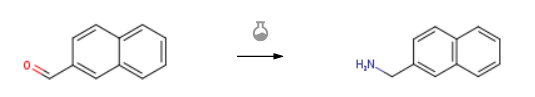
| With ammonia; hydrogen In methanol at 120℃; under 15001.5 Torr; for 4h; Autoclave; Experimental Procedure A method to catalyze the reductive amination of aldehydes and ketones to prepare primary amines, except that “benzaldehyde” in step 2) of Example 1 is replaced with “2-naphthaldehyde” and “reaction at 90°C for 4h” is replaced with “120°C” Except for the reaction for 4h”, everything else was exactly the same as in Example 1. The yield of 2-naphthylmethylamine was 99%. | 99% |
| With ammonium hydroxide; hydrogen In ethanol at 130℃; under 7500.75 Torr; for 12h; Autoclave; Experimental Procedure 2.4. General procedure of the reductive amination General procedure: The reductive amination of carbonyl compounds was performed in a 50 mL stainless steel autoclave reactor. In a typical run, benzaldehyde (1 mmol), Co(at)NC-800 (20 mg), ethanol (8 mL) and NH3.H2O (26.5 wt%, 2 mL) were charged into the reactor, and then the autoclave reactor was closed. The reactor was flushed with H2 for several times to remove air, and then charged with 1 MPa H2 at room temperature. The reaction was then carriedout at 130 °C for 12 h with a stirring rate of 1000 RPM. After reaction,the reaction mixture was cooled down to room temperature and then depressurized. Then, the products in the reaction mixture were detected by gas chromatography by the use of ethylbenzene as the internal standard. The products were also identified by GC/MS (Shimadzu GCMS-QP2010) equipped with Agilent capillary column DB-5MS. | 93.6% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H302 (50%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H411 (50%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store at around 0 ℃ |
| HS Code | |
| Storage | Store at around 0 ℃ |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 157.215 |
| logP | 2.41 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.02 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2-(Aminomethyl)naphthalene CAS 2018-90-8 is mainly used as an intermediate in pharmaceutical synthesis and organic chemistry for producing functional compounds. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |