1-Ethoxy-2,2-Difluoroethan-1-ol CAS#: 148992-43-2; ChemWhat Code: 1411161
Identification
| Product Name | 1-Ethoxy-2,2-Difluoroethan-1-ol |
| IUPAC Name | 1-ethoxy-2,2-difluoroethanol |
| Molecular Structure | 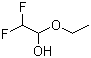 |
| CAS Registry Number | 148992-43-2 |
| EINECS Number | |
| MDL Number | MFCD01321160 |
| Beilstein Registry Number | |
| Synonyms | 1-Ethoxy-2,2-difluoroethanol 148992-43-2 Difluoroacetaldehyde Ethyl Hemiacetal 1-ethoxy-2,2-difluoroethan-1-ol MFCD01321160 SCHEMBL635097 1-Ethoxy-2,2-difluoro-ethanol DTXSID40374237 difluoroacetaldehyde ethylhemiacetal difluroacetaldehyde ethyl hemiacetal Ethanol, 1-ethoxy-2,2-difluoro- BBL103964 STL557774 AKOS015838744 AM803241 AS-48264 SY007828 CS-0179771 D2523 FT-0691024 EN300-64372 F30106 992D432 J-008539 |
| Molecular Formula | C4H8F2O2 |
| Molecular Weight | 126.1 |
| InChI | InChI=1S/C4H8F2O2/c1-2-8-4(7)3(5)6/h3-4,7H,2H2,1H3 |
| InChI Key | WEEOMNFWRCDRJI-UHFFFAOYSA-N |
| Canonical SMILES |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2017/128536 | MANUFACTURING METHOD OF α,α-DIFLUORO ACETALDEHYDE | 2017 |
| CN107438592 | Preservation method of alpha, alpha-difluoroacetaldehyde alkyl hemiacetal | 2017 |
| JP2016/33114 | METHOD FOR IMPROVING STORAGE STABILITY OF 2,2-DIFLUOROACETALDEHYDE | 2016 |
| US2013/79324 | PYRROLOPYRIMIDINE AND PURINE DERIVATIVES | 2013 |
Physical Data
| Appearance | Colorless to light yellow liquid |
| Melting Point, °C | Solvent (Melting Point) |
| No data available |
| Boiling Point, °C |
| 45 – 47 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 150 | |
| Chemical shifts | 1H | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | KBr |
Route of Synthesis (ROS)
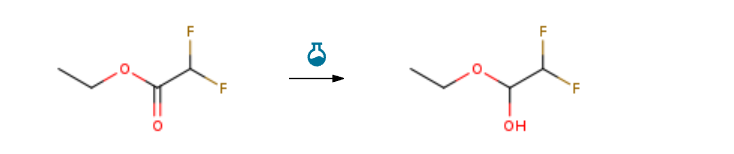
Route of Synthesis (ROS) of 1-ethoxy-22-difluoroethan-1-ol-cas-148992-43-2
| Conditions | Yield |
| With lithium aluminium tetrahydride In tetrahydrofuran; diethyl ether at -78℃; for 2.75h; | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran at -78℃; for 4.5h; Time; Inert atmosphere; | 84% |
| With hydrogen In 2-methyltetrahydrofuran; water at 40℃; under 15001.5 Torr; for 24h; chemoselective reaction; | 98% |
| Experimental Procedure Scheme 4, steps A and B: To an oven dried 1 L flask is added ethy1 2,2- difluoroacetate (25.4 mL, 242 mmol) and diethy1 ether (40 mL), and the mixture is cooled to -78 °C. To the mixture is added lithium aluminum hydride (1 M solution in THF, 61.0 mL, 61.0 mmol) dropwise via addition funnel over a period of 25 minutes. The addition funnel is further washed with diethy1 ether (5 mL) and added dropwise. After 2 hours and 45 minutes, the reaction is quenched with the slow addition of EtOH (6 mL), and is allowed to stir at room temperature for 20 minutes. The mixture is then poured into a mixture of concentrated sulfuric acid (15 mL) in crushed ice (200 mL). After stirring for 5 minutes, the mixture is diluted with diethy1 ether (150 mL), poured into a separatory funnel, and the layers are separated. The aqueous layer is extracted once more with diethy1 ether (200 mL), and the combined organics are dried over magnesium sulfate, filtered, and concentrated in vacuo to give the intermediate 1 -ethoxy-2, 2-difluroethanol in a volume of 45 mL with assumed quantitative yield.erimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | 97% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H226 (100%): Flammable liquid and vapor [Warning Flammable liquids] H315 (18.18%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (18.18%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (27.27%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | Half of a year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 126.103 |
| logP | 0.817 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 29.46 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Quantitative Results No data available |
| Use Pattern |
| 1-Ethoxy-2,2-Difluoroethan-1-ol CAS#: 148992-43-2 is used in pharmaceutical intermediates. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to [email protected] |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

