1,2,3,4,6-Penta-O-benzoyl-alpha-D-mannopyranose CAS#: 41569-33-9; ChemWhat Code: 1397578
Identification
| Product Name | 1,2,3,4,6-Penta-O-benzoyl-alpha-D-mannopyranose |
| IUPAC Name | (3,4,5,6-tetrabenzoyloxyoxan-2-yl)methyl benzoate |
| Molecular Structure | 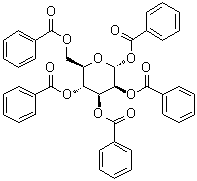 |
| CAS Registry Number | 41569-33-9 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | 1,2,3,4,6-penta-O-benzoyl-D-glucopyranoside, 1,2,3,4,6-penta-O-benzoyl α-D-glucopyranoside, 1,2,3,4,6-penta-O-benzyl-β-D-glucopyranoside, benzoyl 2,3,4,6-tetra-O-benzoyl-D-glucopyranoside, 1,2,3,4,6-penta-O-benzoyl-D-glucopyranose, penta-O-benzoyl-D-glucopyranose, per-O-benzoylated glucopyranose |
| Molecular Formula | C41H32O11 |
| Molecular Weight | 700.69 |
| InChI | InChI=1S/C41H32O11/c42-36(27-16-6-1-7-17-27)47-26-32-33(49-37(43)28-18-8-2-9-19-28)34(50-38(44)29-20-10-3-11-21-29)35(51-39(45)30-22-12-4-13-23-30)41(48-32)52-40(46)31-24-14-5-15-25-31/h1-25,32-35,41H,26H2 |
| InChI Key | JJNMLNFZFGSWQR-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC=C(C=C1)C(=O)OCC2C(C(C(C(O2)OC(=O)C3=CC=CC=C3)OC(=O)C4=CC=CC=C4)OC(=O)C5=CC=CC=C5)OC(=O)C6=CC=CC=C6 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2016/264609 | DISACCHARIDE INTERMEDIATE AND SYNTHESIS METHOD THEREOF | 2016 |
Physical Data
| Appearance | White to off-white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C |
| 172 – 174 |
Spectra
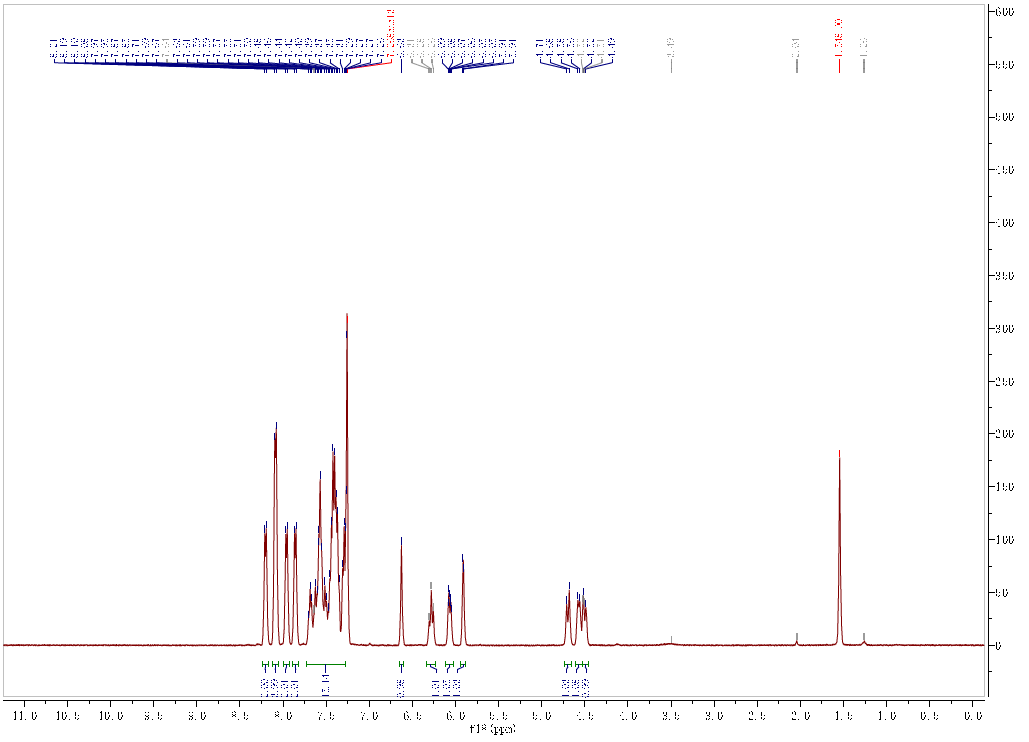
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 599.9 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 150.8 |
| 1H | chloroform-d1 | ||
| Chemical shifts | 1H | CDCl3 | 300 |
| Chemical shifts | 13C | CDCl3 | 75 |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) | Peak |
| ESI (Electrospray ionisation), HRMS (High resolution mass spectrometry) | Molecular peak | 723.1818 m/z |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogen bromide; acetic acid In dichloromethane at 0 – 20℃; for 2h; Experimental Procedure Step (b) [00245] To a solution of 1,2,3,4,6-penta-O-benzoyl-D-glucopyranose (2.5 g, 3.57 mmol) in anhydrous CH2C12 at 0 °C was added HBr solution in AcOH (33%, 10 mL) and stirred for 1 hr. The temperature was then increased to room temperature and stirred for another hour. The solvent was removed under vacuum and the residue was dissolved in CH2C12 (100 mL) and neutralized with saturated aqueous NaHC03 (50 mL). The organic layer was separated and washed with H20 (3 x 50 mL), saturated aqueous NaHC03 (3 x 30 mL) and brine (2 x 30 mL). Then the solution was dried with anhydrous Na2S04, filtered and concentrated in vacuum to afford 2,3,4,6-tetra-O-benzoyl-a-D-glucopyranosyl bromide quantitatively as a white foamy solid | 100% |
| With hydrogen bromide In acetic anhydride; acetic acid at 50℃; for 4.5h; | 97% |
| With hydrogenchloride; hydrogen bromide In 1,2-dichloro-ethane at 20℃; for 2h; Cooling with ice; Experimental Procedure Reagents and reaction conditions: benzoyl chloride, pyridine, room temperature; () 33% hydrobromic acid in acetic acid, 1,2-dichloroethane, room temperature. Preparation steps: (i) taking D- glucose (. 15g, 83 26mmol) in the reaction flask was added 150mL of anhydrous pyridine, stirred at rt for 30min, was added dropwise benzoyl chloride (60mL, 520.7mmol) under ice-water bath cooled sufficiently. Completion of the dropwise addition, stirring at room temperature for 15min, then the reaction was continued at 60 ° C 2h. The reaction solution was poured into ice water, stand until after curing, and the filter cake was washed successively with dilute hydrochloric acid, water, and methanol, and dried to obtain a white powder sample S-1 (53. 47g, yield 91.7%) . (ii) to take S-l (20g, 28. 54mmol) in the reaction flask was added lOOmLl, 2- dichloroethane was added dropwise 33% hydrobromic acid / acetic acid solution (80mL, 463. 3mmol) under an ice-water bath cooled sufficiently. Completion of the dropwise addition, then stirred at room temperature for 2h. The reaction solution was poured into a mixture of ethyl acetate and ice water, extracted organic phase was washed successively with saturated aqueous sodium bicarbonate and water, dried over anhydrous magnesium sulfate, and ethyl acetate recovered under reduced pressure, the resulting residue was 300mL cyclohexane dispersion, ultrasound, a solid, the filter cake dried to obtain a white powder of 2,3,4,6-tetrabenzoyl-(1-bromo-glucopyranose (S-02) 17.48 g, yield 92%. | 92% |
Safety and Hazards
| No data available |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data availabe |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 700.698 |
| logP | 8.872 |
| HBA | 11 |
| HBD | 0 |
| Matching Lipinski Rules | 1 |
| Veber rules component | |
| Polar Surface Area (PSA) | 140.73 |
| Rotatable Bond (RotB) | 16 |
| Matching Veber Rules | 0 |
| Use Pattern |
| 1,2,3,4,6-Penta-O-benzoyl-alpha-D-mannopyranose CAS#: 41569-33-9 used as pharmaceutical intermediates. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |