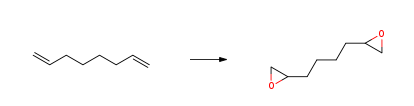
1,2,7,8-DIEPOXYOCTANE CAS#: 2426-07-5; ChemWhat Code: 73196
Identification
| Product Name | 1,2,7,8-DIEPOXYOCTANE |
| IUPAC Name | 2-[4-(oxiran-2-yl)butyl]oxirane |
| Molecular Structure | 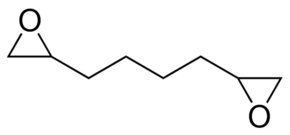 |
| CAS Registry Number | 2426-07-5 |
| EINECS Number | 219-375-9 |
| MDL Number | MFCD00005155 |
| Synonyms | 1,2,7,8-diepoxyoctane, 2-(4-(oxiran-2-yl)butyl)oxirane, 1,2,7,8-Octadiene bisepoxide, 1,4-di(oxiran-2-yl)butane, Octa-1,7-diene diepoxide, octane-1,2,7,8-diepoxide, 1,7-octadiene diepoxide;CAS#: 2426-07-5;CAS No. 2426-07-5;1,2,7,8-DIEPOXYOCTANE CAS Number: 2426-07-5 |
| Molecular Formula | C8H14O2 |
| Molecular Weight | 142.196 |
| InChI | InChI=1S/C8H14O2/c1(3-7-5-9-7)2-4-8-6-10-8/h7-8H,1-6H2 |
| InChI Key | LFKLPJRVSHJZPL-UHFFFAOYSA-N |
| Canonical SMILES | C1C(O1)CCCCC2CO2 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP2602251 | METHOD FOR PRODUCING EPOXY COMPOUND BY OXIDATION | 2013 |
| US5045230 | Thickening agents for aqueous systems | 1991 |
| US5679617 | Agent and method for preserving freshness of cut flowers | 1997 |
| US4628102 | Novel monomers containing bicyclic amide acetal and epoxy functional groups | 1986 |
Physical Data
| Appearance | Colorless liquid |
| Solubility | 7g/l |
| Refractive index | n20/D 1.445(lit.) |
| Water soluble | Soluble in water (partly miscible), acetone and heptane. |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 101 | 7 |
| 120 | 28 |
| Description (Adsorption (MCS)) | Partner (Association (MCS)) |
| Chemisorption | silicon |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 13C | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | 300 | |
| Chemical shifts | 13C | chloroform-d1 | 75 | |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 1H | CDCl3 | 25 | |
| NMR with shift reagents |
| Description (Mass Spectrometry) |
| GCMS (Gas chromatography mass spectrometry), Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; Experimental Procedure 4.5. 1,7-Octadiene dioxide (8) Octadiene (1.37 mL, 9.07 mmol) was dissolved in 25 mL of DCM and stirred at 0 °C. MCPBA (4.68 g, 18.08 mmol) dissolved in 45 mL of DCM was added dropwise. The solution was left at 0 °C under stirring overnight. The reaction was monitored by TLC (PE/EtOAc 8:2) and spots were visualized with a phosphomolybdic acid stain. The solution was washed three times with water and finally dried (Na2SO4). 1.39 g (95% yield) of the desired product was recovered and employed without further purifications. Analytical data were in accordance with reported values.refPreviewPlaceHolder20 E.W. Meijer, A.R.A. Palmans, B.A.C. van As, J. van Buijtenen and T. Mes. Chem. Eur. J., 13 (2007), pp. 8325-8332.?20 | 95% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 – 20℃; for 24h; | 94% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 – 20℃; for 2h; | 83% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H311 (100%): Toxic in contact with skin [Danger Acute toxicity, dermal] H341 (97.73%): Suspected of causing genetic defects [Warning Germ cell mutagenicity] H350 (88.64%): May cause cancer [Danger Carcinogenicity] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P264, P270, P280, P281, P301+P312, P302+P352, P308+P313, P312, P322, P330, P361, P363, P405, and P501 (The corresponding statement to each P-code can be found at the?GHS Classification?page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: III; UN Number: 2810 |
| Under the room temperature and away from light | |
| HS Code | 291090 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 142.198 |
| logP | 0.916 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 25.06 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Parameter | Value (qual) | Value (quant) | Unit |
| Activity(Carcinogenic activity) | ND | ||
| Vmax | = | 3.31 | mM/min |
| Km (Michaelis constant) | = | 300.8 | mM |
| Quantitative Results | ||
| 1?of?9 | Target | Lipase B, Lysosomal Acid [Candida antarctica]:Wild |
| Biological material | Candida antarctica | |
| Assay Description | Enzyme activity of Candida antarctica lipase B upon incubation with compound in the presence of Ammonium sulfate | |
| Results | ENZYMATIC ACTIVITY not calculated | |
| Measurement | enzymatic activity | |
| 2?of?9 | Target | Lipase B, Lysosomal Acid [Candida antarctica]:Wild |
| Biological material | Candida antarctica | |
| Assay Description | Enzyme activity of Candida antarctica lipase B upon incubation with compound in the presence of Ammonium sulfate and Triton X-100 | |
| Results | ENZYMATIC ACTIVITY not calculated | |
| Measurement | enzymatic activity | |
| 3?of?9 | Target | Lipase B, Lysosomal Acid [Candida antarctica]:Wild |
| Biological material | Candida antarctica | |
| Assay Description | Maximum velocity of the compound towards Candida antarctica lipase B with respect to Michaelis constant | |
| Results | VMAX/KM not calculated | |
| Measurement | Vmax/Km | |
| 4?of?9 | Target | Lipase B, Lysosomal Acid [Candida antarctica]:Wild |
| Biological material | Candida antarctica | |
| Assay Description | Enzyme activity of Candida antarctica lipase B upon incubation with compound in the presence of 1,2-dimethoxyethane and 18-crown-6 | |
| Results | ENZYMATIC ACTIVITY not calculated | |
| Measurement | enzymatic activity | |
| 5?of?9 | Target | Lipase B, Lysosomal Acid [Candida antarctica]:Wild |
| Biological material | Candida antarctica | |
| Assay Description | Enzyme activity of Candida antarctica lipase B upon incubation with compound in the presence of 1,2-dimethoxyethane | |
| Results | ENZYMATIC ACTIVITY not calculated | |
| Measurement | enzymatic activity | |
| 6?of?9 | Target | Lipase B, Lysosomal Acid [Candida antarctica]:Wild |
| Biological material | Candida antarctica | |
| Assay Description | Enzyme activity of Candida antarctica lipase B upon incubation with compound in the presence of Ammonium sulfate and 18-crown-6 | |
| Results | ENZYMATIC ACTIVITY not calculated | |
| Measurement | enzymatic activity | |
| 7?of?9 | Effect | Carcinogenic |
| Assay Description | Target : radiolabeled duplex DNA Bioassay : interstrand cross-linking capability of title comp. with respect to chain lenght, molecular flexibility, reported carcinogenic potential and DNA sequences targeted, examined using denaturing polyacrylamide gel electrophoresis and autoradiography title comp. incubated with DNA (0.5 D;260) in sodium acetate (0.3 mmol/l, pH 5) for 2 h at 37 deg C | |
| Results | title comp. had 5′-GNC target sequence; efficiency of cross-linking (table) may reflect carcinogenicity | |
| 8?of?9 | Effect | Carcinogenic |
| Assay Description | Target : radiolabeled duplex DNA Bioassay : monoalkylation of the GGGGCGGG duplex and average relative percentages of monoaduct formation as a function of nucleotide position determined title comp. incubated with DNA (0.5 D;260) in sodium acetate (0.3 mmol/l, pH 5) for 2 h at 37 deg C | |
| Results | percent of cleavage at G1 – G6 and total alkylation in table | |
| 9?of?9 | Assay Description | Effect : DNA; examination of Target : human lung epithelial carcinoma cells A549 Bioassay : 400000 cells/ml, incubated 37 and 50 deg C in saline, pulsed-field gel electrophoresis, DNA double-strand break (DSB) assessed, cell viability determination |
| Results | test comp. induced DSB at 8 h, markedly different fraction of DNA (activity) released values obtained at 37 and 50 deg C, DNA-DNA crosslinks 65 percent with 300 μM, reduction of cell viability 30 percent, considerable repair of crosslinks observed |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 1 | inhibition rate | 12.5 | % | ||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | 10 | % | ||
| 1 | inhibition rate | Not active | |||
| 1 | inhibition rate | Not active | |||
| 1 | CC50 (cytotoxic concentration) | > | 1060 | μM | Cytotoxic |
| 1 | CC90 | > | 1060 | μM | Cytotoxic |
| Use Pattern |
| 1,2,7,8-DIEPOXYOCTANE CAS#: 2426-07-5 as crosslinking agent for preparing a crosslinked hyaluronic acid |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |