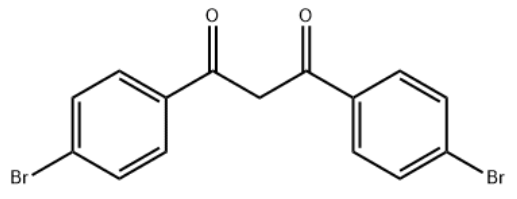
1,3-bis(4-bromophenyl)propane-1,3-dione CAS #: 33170-68-2; ChemWhat Code: 1490994
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN115093323 | Beta-functionalized chiral homoallylic alcohol derivative as well as preparation method and application thereof | 2022 |
| CN112679447 | Preparation method of polysubstituted thiazole-2(3H)-ketone compound | 2021 |
| CN105348060 | Preparation method of 1,2-diketone derivative | 2016 |
| CN105503945 | Method for preparing 2-phosphonic acid ester base-1, 3-dicarbonyl derivative | 2016 |
| US2006/69288 | Process for preparing vinyl substituted beta-diketones | 2016 |
Physical Data
| Appearance | White solid |
| Melting Point, °C | Solvent (Melting Point) |
| 198 – 200 | ethanol |
| 191.5 – 194.5 | |
| 183 | |
| 185 – 186 | toluene |
| 195 – 196 | ethanol |
| 196 – 198 | benzene |
| 197 – 198.5 | benzene |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | chloroform-d1 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Spectrum | 13C | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Spectrum | potassium bromide | |
| Bands | KBr | 1600 cm**(-1) |
| Spectrum | CHCl3 | 1750 – 1450 cm**(-1), der bei 194grad, 183grad und 167grad schmelzenden Formen. |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | ethanol | Ratio of solvents: 66percent | 350.8, 265.8 |
| Spectrum | dioxane | 220 – 380 nm, der bei 194grad, 183grad und 167grad schmelzenden Formen. |
Route of Synthesis (ROS)
| Conditions | Yield |
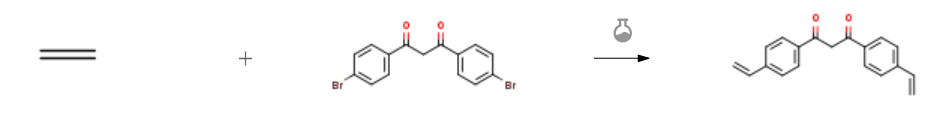
| With palladium diacetate; triethylamine; tris-(o-tolyl)phosphine In N,N-dimethyl-formamide at 100℃; under 15514.4 Torr; for 12h; Heck coupling; | 62% |
| With triethylamine; palladium diacetate; tris-(o-tolyl)phosphine In N,N-dimethyl-formamide at -196 – 100℃; under 15514.9 Torr; for 12.3333h; Experimental Procedure 2.4 Step 4 Synthesis of 4,4′-divinyldibenzoylmethane: Dimethyl formamide (30 mL), palladium acetate (0.022 g, 0.1 mmol), tri-o-tolyl phosphine (0.060 g, 0.2 mmol), triethylamine (9.1 g, 90 mmol), 4,4′-dibromodibenzoylmethane (3.82 g, 10 mmol) were added to a 100 mL Parr high pressure reactor with an inner glass liner. The solution was degassed with nitrogen for 10 minutes before the reactor was cooled to -196° C. and ethylene (1.7 g, 61 mmol) was condensed for 20 minutes. The reactor was allowed to warm to room temperature before heating to 100° C. in an oil bath. The pressure was bled off until 300 psi was achieved and the reaction was allowed to proceed for 12 hours. The reaction vessel was cooled to room temperature, the extra pressure bled, and the contents dissolved in water (50 mL) and ether (50 mL). The aqueous phase was made acidic by addition of concentrated hydrochloric acid followed by extraction with ether (2*50 mL). The combined organic phase was rinsed with saturated sodium chloride solution, and the organic phase was dried over magnesium sulfate overnight. The solution was filtered, the solvent was removed by vacuum, and the residue dissolved in chloroform (3 mL). The product was isolated by column chromatography from silica gel (Selecto, mesh size 63 – 200) with chloroform as eluent. The first band collected resulted in a yellow powder (1.70 g, 62% yield) upon solvent removal. 1H-NMR (200 MHz, 25° C., CDCl3): δ 16.20 (s, 1 H), 8.00 (d, 4 H), 7.55 (d, 4 H), 6.80 (dd, 2 H), 6.70 (s, 1 H), 5.90 (d, 2 H), 5.40 (d, 2 H). | 62% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (100%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P264+P265, P273, P280, P305+P351+P338, P337+P317, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 382.051 |
| logP | 5.255 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 34.14 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 3-Aminopyridine CAS#: 462-08-8 is an intermediate in pesticides and dyes; pesticide raw materials; analytical reagents. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | https://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |