1,7-Octadiene CAS#: 3710-30-3; ChemWhat Code: 32282
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117924126 | Method for hydrogen amine alkylation of aliphatic olefin under catalysis of nickel | 2024 |
| CN110981836 | Method for preparing diepoxide by one-pot method | 2020 |
| CN103588807 | A double ( alkane oxygen silicon-based ) alkane preparation method | 2016 |
| EP2602251 | METHOD FOR PRODUCING EPOXY COMPOUND BY OXIDATION | 2013 |
| US2012/29229 | DIAZABICYCLOOCTANE DERIVATIVES COMPRISING A QUATERNERY AMMONIUM GROUP FOR USE AS ANTIBACTERIAL AGENTS | 2012 |
| US2005/107626 | Alkylidene complexes of ruthenium containing N-heterocyclic carbene ligands; use as highly active, selective catalysts for olefin metathesis | 2005 |
Physical Data
| Appearance | Colorless to light yellow transparent liquid |
| Melting Point, °C | Solvent (Melting Point) |
| -111.16 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 117 | 760.051 |
| 113.99 | 750.075 |
| 117 – 119 | |
| 115 | |
| 117 | |
| 124 | 760 |
| 57 – 57.5 | 92 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.925 | -123.16 | |
| 0.72708 | 25 | |
| 0.7202 – 0.8034 | -68.8 – 29.9 | |
| 0.7289 | 25 | |
| 0.7313 | 20 | |
| 0.7301 | 4 | 20 |
| 0.7293 | 4 | 21.4 |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| Adsorption | zirconium(IV) oxide |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | benzene-d6 | 24.84 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 24 | |
| Chemical shifts | 1H | benzene-d6 | ||
| Chemical shifts, Spectrum | 1H | tetrahydrofuran-d8 | 300 | |
| Chemical shifts | 1H | |||
| Chemical shifts | 1H | chloroform-d1 | ||
| Chemical shifts | 13C | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | |
| Spectrum | gas |
| Bands | neat (no solvent) |
Route of Synthesis (ROS)
| Conditions | Yield |
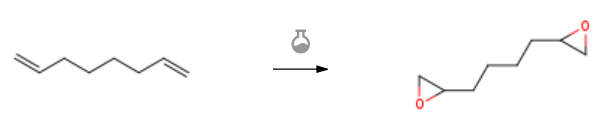
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; Experimental Procedure 4.5. 1,7-Octadiene dioxide (8) Octadiene (1.37 mL, 9.07 mmol) was dissolved in 25 mL of DCM and stirred at 0 °C. MCPBA (4.68 g, 18.08 mmol) dissolved in 45 mL of DCM was added dropwise. The solution was left at 0 °C under stirring overnight. The reaction was monitored by TLC (PE/EtOAc 8:2) and spots were visualized with a phosphomolybdic acid stain. The solution was washed three times with water and finally dried (Na2SO4). 1.39 g (95% yield) of the desired product was recovered and employed without further purifications. Analytical data were in accordance with reported values.refPreviewPlaceHolder20 E.W. Meijer, A.R.A. Palmans, B.A.C. van As, J. van Buijtenen and T. Mes. Chem. Eur. J., 13 (2007), pp. 8325-8332. 20 | 95% |
| With 3-chloro-benzenecarboperoxoic acid at 10 – 23℃; for 16h; Experimental Procedure 2 A one-pot method for preparing diepoxide includes the following steps: S1. Epoxidation reaction: At low temperature, add 12.69g of 1,7-octadiene to the reactor, slowly add m-chloroperoxybenzoic acid solution dropwise, control the drop acceleration to keep the system temperature at about 10 , and maintain after the dropwise addition The reaction was carried out at a low temperature for 4 hours, then the temperature was raised to 23 ° C., the reaction was continued for 12 hours, and the reaction was completed to obtain a crude dioctane solution, in which the molar ratio of diene to m-chlorobenzoyl chloride was 1: 2.6;S2. Separation and purification: add 220g of 20% mass fraction sodium bisulfite aqueous solution to the crude diepoxyoctane solution, keep the system temperature at 23°C, reduce the reaction for 2h, extract and separate the organic phase. Add 220g mass fraction 20% sodium carbonate solution to the organic phase, maintain the system at 23 , neutralize the reaction for 2h, extract and separate the organic phase. The organic phase was washed twice with water (160 mL×2), the organic phase was extracted and separated, dried over anhydrous magnesium sulfate, the solid filter residue was filtered off, the filtrate was distilled under reduced pressure, the solvent dichloroethane was recovered, and the product was collected to obtain diepoxyoctane .Among them, the preparation method of m-chloroperoxybenzoic acid solution is as follows:Add 36g of sodium hydroxide, 260mL of water, 120g of 30% hydrogen peroxide solution, 2.0g of magnesium sulfate heptahydrate to the reactor, stir to dissolve, then add 250mL of dioxane, control the feeding temperature at 15 , under vigorous stirring Next, 52.5g of m-chlorobenzoyl chloride was added at one time, maintaining the reaction temperature at 25°C, and stirring to react for 15 min. Reduce the temperature, add 20% sulfuric acid solution, control the temperature of the system at 10, acidify to pH 3, add 300mL of dichloroethane, extract and separate the organic phase, dry with anhydrous potassium sulfate, filter off the solid filter residue to get m-chloride Oxybenzoic acid/dichloroethane solution.The molar ratio of chlorobenzoyl chloride, sodium hydroxide and hydrogen peroxide is 1:3:3.53.The yield of diepoxyoctane is 95.2% and the purity is 91.7%. | 95.2% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 – 20℃; for 24h; | 94% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H225 (100%): Highly Flammable liquid and vapor [Danger Flammable liquids] H304 (99.1%): May be fatal if swallowed and enters airways [Danger Aspiration hazard] H400 (42.2%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (42.2%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] H412 (56%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P273, P280, P301+P316, P303+P361+P353, P331, P370+P378, P391, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 110.199 |
| logP | 4.16 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Limited | http://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |