1,9-DECADIENE CAS#: 1647-16-1; ChemWhat Code: 32342
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/35451 | Solid State Polymerization Process for Polyester with Phosphinic Acid Compounds | 2013 |
| US2014/155666 | PALLADIUM-CATALYZED DECARBONYLATION OF FATTY ACID ANHYDRIDES FOR THE PRODUCTION OF LINEAR ALPHA OLEFINS | 2014 |
Physical Data
| Melting Point, °C |
| -77.16 |
| -71.8 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 68 | 20 |
| 98 – 99 | 90.009 |
| 108 – 110 | 125 |
| 80 | 40 |
| 165 – 170 | |
| 165 | 760 |
| 167 | 760 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.754 | 20 | |
| 0.946 | -123.16 | |
| 0.7602 | 4 | 20 |
| 0.755 | 20 | |
| 0.7478 | 4 | 28 |
| 0.7534 | 4 | 20 |
| 0.7484 | 4 | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | gas |
Route of Synthesis (ROS)
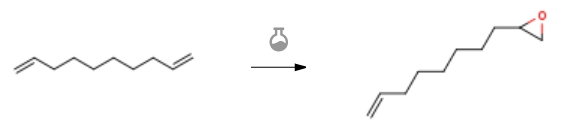
| Conditions | Yield |
| With oxygen In acetonitrile at 60℃; under 760.051 Torr; for 1.5h; Catalytic behavior; chemoselective reaction; Experimental Procedure General procedure: Epoxidation of olefins was carried out in a 25-mL three-necked round-bottom flaskequipped with O2 gas (as an oxidant) inlet (*1 atm., bubbling 15 mL/min), watercondenser and magnet stirrer bar. In a typical run, a mixture of alkene (10 mmol)and 50 mg of catalyst 4 (2 mol%) were added to acetonitrile (20 mL) and thereaction was kept in a constant temperature oil bath at 60 C. Molecular oxygenwith a rate of 15 mL/min-1 was bubbled during the reaction. The reaction mixture was stirred vigorously for a sufficient time. | 85% |
| With p-chloroperbenzoic acid In dichloromethane | |
| With oxygen In acetonitrile under 760.051 Torr; for 1h; Kinetics; Experimental Procedure 2.6. General procedure for catalytic epoxidation of olefins by GO/Fe3O4(at)PAA-Co(II) General procedure: In a typical run, in a three-necked round-bottom flask equippedwith a O2 (g) inlet (bubbling 15 mL/min, 1.0 atm.), a condenser and a magnet stirrer bar, a mixture of styrene (10.0 mmol) and catalyst 8 (50 mg, 0.2 mol%) were stirred in CH3CN (10 mL) at 60 °C. The reaction progress was monitored by TLC. Upon reaction completion, the catalyst was magnetically filtered, and the residue was directly subjected to GC instrument to quantify the epoxide product. Moreover, the desired epoxide product was purified by column chromatography followed by identification by 1H NMR and 13CNMR spectra. Activity of the catalyst was expressed by turnover frequency (TOF) as well as turnover number (TON) [1,5]. | 90 %Chromat. |
| With hydrogen; triethylamine In ethanol; water at With dihydrogen peroxide; C15H18N9(3+)3O5S(2-)3H(1+) at 40℃; Reagent/catalyst; Irradiation; Experimental Procedure 2.3. General procedure for ultrasound-promoted TAIm[X] (X = WO4,HSO5) IL-catalyzed olefins epoxidation General procedure: Alkene (10 mmol), H2O2 30% (1.5 mL, 19.2 mmol), and 0.5 mL ofTAIm[X] (X = WO4, HSO5) IL were added to a 10 mL round bottom flask.The flask was placed in a ultrasonic bath and the temperature bath wasadjusted to 40 °C. The mixture was ultrasonicated for appropriate timethat was monitored by GC instrument at various time intervals. Conversionand selectivity of the epoxide products were measured accordingto the following equations (1) and (2) respectively [1,2] after the recoveryof IL3 (or IL4): conversion (mol%) =(initial mol%) – (final mol%)initial mol%× 100 (1)Epoxide selectivity =GC peak area of the desired epoxideGC peak area of all products× 100 (2) To recycle of TAIm[X] (X = WO4, HSO5) IL, 2.0 mL distilled water and 2.0 mL of CHCl3 was added to the mixture reaction. The aqueouslayer containing TAIm[X] (X = WO4, HSO5) IL was separated andrecovered after removal of water under reduced pressure. Finally, the ILwas stored at 4 C in the presence of Molecular Sieve UOP Type 3 Å(MERCK, beads, 8-12 mesh) for the next use.In order to purify the epoxide products, the organic phase containingthe epoxide product was extracted several times with ether afterremoving the previous solvent [22]. In some cases, the column chromatographywas used for further purification. | 97 %Chromat. |
| With dihydrogen peroxide; C15H18N9(3+)3O5S(2-)3H(1+) at 40℃; Reagent/catalyst; Sonication; Experimental Procedure 2.3. General procedure for ultrasound-promoted TAIm[X] (X = WO4,HSO5) IL-catalyzed olefins epoxidation General procedure: Alkene (10 mmol), H2O2 30% (1.5 mL, 19.2 mmol), and 0.5 mL ofTAIm[X] (X = WO4, HSO5) IL were added to a 10 mL round bottom flask.The flask was placed in a ultrasonic bath and the temperature bath wasadjusted to 40 °C. The mixture was ultrasonicated for appropriate timethat was monitored by GC instrument at various time intervals. Conversionand selectivity of the epoxide products were measured accordingto the following equations (1) and (2) respectively [1,2] after the recoveryof IL3 (or IL4): conversion (mol%) =(initial mol%) – (final mol%)initial mol%× 100 (1)Epoxide selectivity =GC peak area of the desired epoxideGC peak area of all products× 100 (2) To recycle of TAIm[X] (X = WO4, HSO5) IL, 2.0 mL distilled water and 2.0 mL of CHCl3 was added to the mixture reaction. The aqueouslayer containing TAIm[X] (X = WO4, HSO5) IL was separated andrecovered after removal of water under reduced pressure. Finally, the ILwas stored at 4 C in the presence of Molecular Sieve UOP Type 3 Å(MERCK, beads, 8-12 mesh) for the next use.In order to purify the epoxide products, the organic phase containingthe epoxide product was extracted several times with ether afterremoving the previous solvent [22]. In some cases, the column chromatographywas used for further purification. |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Warning |
| GHS Hazard Statements | H226 (100%): Flammable liquid and vapor [Warning Flammable liquids] H315 (84.75%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (84.75%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H400 (91.53%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (15.25%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P271, P273, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P391, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 138.253 |
| logP | 5.298 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 7 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Synthesis of diols, for use as intermediates for polymeric materials, by starting from terminal diolefins | |
| 2 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 3 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | TETRABUTYLAMMONIUM BIFLUORIDE: A VERSATILE AND EFFICIENT FLUORINATING AGENT | |
| 4 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 5 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Acrylonitrile cross-metathesis: Coaxing olefin metathesis reactivity from a reluctant substrate |
| Use Pattern |
| Due to its unsaturated double bond structure, 1,9-decadiene can participate in polymerization reactions to manufacture synthetic rubbers with specific properties. 1,9-decadiene can be used as a comonomer to copolymerize with other monomers to form plastics and resins with desired characteristics. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

