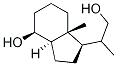
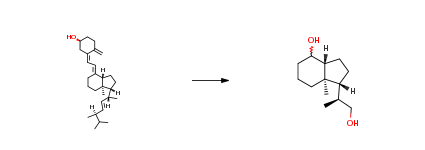
| Conditions | Yield |
| With sodium tetrahydroborate; ozone In methanol; dichloromethane at -78℃ | 75% |
Stage #1: Calciferol With sodium hydrogencarbonate; ozone In methanol; dichloromethane at -78℃;
Stage #2: With sodium tetrahydroborate In methanol; dichloromethane at -78 – 0℃;
Experimental Procedure
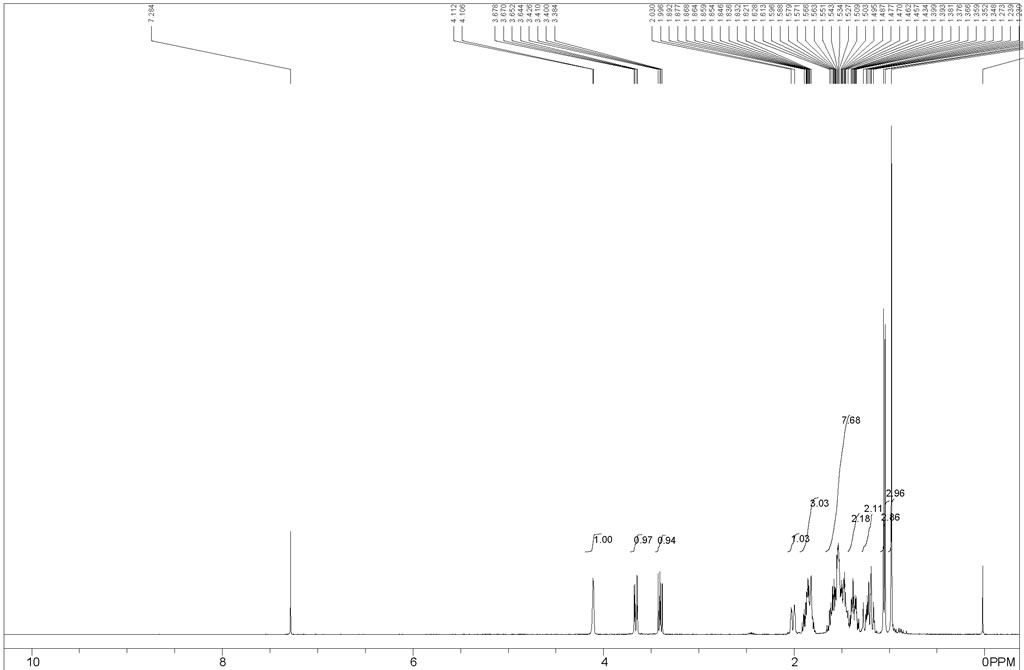
To an cold (-78 °C) solution of vitamin D2 (ergocalciferol, 25 g, 63.07 mmol) in MeOH:CH2Cl2 (250 mL:750 mL) was added solid NaHCO3 (0.5 g) and the resulting solution and treated with O3 until a deep blue color developed and persisted. The solution was subsequently flushed with Argon for 10-15 min until the blue color faded. Solid NaBH4 (12.5 g, 327.75 mmol) was added portion wise over a period of 10 min at -78 °C and then the mixture was warmed to 0 °C and then warmed to room temperature and stirred for 3 h. The reaction mixture was quenched with 2N HCl, extractedwith EtOAc, dried over Na2SO4, concentrated under reduced pressure to get the crude product. The residue was purified by silica gel column chromatography (20-25percent EtOAc in pet ether) afforded 6 g (60percent) as a white solid. Rf 0.5 (40percent EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) 4.08 (br s, 1H), 3.65-3.61 (dd, J = 10.4, 3.2 Hz, 1H), 3.40-3.35 (dd, J = 10.4, 6.4 Hz, 1H), 2.0-1.97 (dd, 12.8Hz, 1H), 1.89-1.77 (m, 3H), 1.64-1.40 (m, 5H), 1.39-1.30 (m, 4H), 122-1.13 (m, 2H), 1.04-1.02 (d,(m, J = 3 Hz, 3H), 0.95 (s, 3H); LC-MS (ES+) m/z 213.4 (M+H). | 60% |
Stage #1: Calciferol With sodium hydrogencarbonate; ozone In methanol; dichloromethane at 20℃;
Stage #2: With sodium tetrahydroborate;
Experimental Procedure
This example describes the synthesis of (IR, 3aR, 7aR)- 1 -((S)- 1 -hydroxypropan-2- yl)-7α-methyloctahydro-lH-inden-4-ol, which is an intermediate of a compound of Formula (I), in an embodiment of the invention.[0288] A flame-dried 250 mL three-necked flask was charged sequentially with 300 mg (3.6 mmol) of sodium bicarbonate, 50.0 mL of anhydrous methanol, 50.0 mL of anhydrous CH2Cl2, and ergocalciferol ((+) vitamin D2, 3.0 g, 7.6 mmol). The solution was treated with O3 (O2 5 g/h) and stirred constantly at room temperature for 4-5 h. Solid sodium borohydride (2.5 g, 64 mmol) was then added portion-wise over a period of 10 min in a water bath until complete disappearance of the starting material was observed by TLC. The resulting reaction mixture was quenched with 4 N hydrochloric acid, extracted with EtOAc (3×30 mL), dried over MgSO4, filtered, and concentrated in vacuo. Purification by silica gel chromatography (30percent EtOAc/petroleum ether) yielded 8.10 g (3.8 mmol) of the title compound in 50percent yield. 1H-NMR (400 MHz5CDCl3): δ 0.98 (s, 3H), 1.05 (d, 3H, J = 6.4 Hz), 1.2 (d, 2H, J = 10.4 Hz), 1.36-1.82 (m,12H), 1.85(d, IH, J = 3.6 Hz), 3.38-3.42 (dd, IH, J = 10.4, 6.8 Hz), 3.64- 3.67(dd, IH, J = 10.4,3.6Hz), 4.10 (d, IH, J = 2.4 Hz). | 50% |
| With ozone | |
Multi-step reaction with 2 steps
1: ozonolysis
2: sodium tetrahydroborate | |