(1S,2S)-2-Fluorocyclopropanecarboxylic acid CAS#: 127199-14-8; ChemWhat Code: 1411675
Identification
| Product Name | (1S,2S)-2-Fluorocyclopropanecarboxylic acid |
| IUPAC Name | (1S,2S)-2-fluorocyclopropane-1-carboxylic acid |
| Molecular Structure | 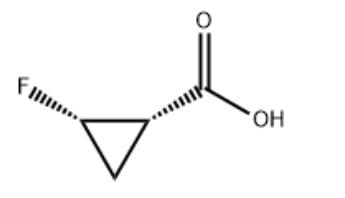 |
| CAS Registry Number | 127199-14-8 |
| Synonyms | (1S,2S)-2-fluorocyclopropanecarboxylic acid 127199-14-8 105919-34-4 cis-2-fluorocyclopropanecarboxylic acid (1s,2s)-2-fluorocyclopropane-1-carboxylic acid cis-2-Fluorocyclopropane-1-carboxylic acid cis-2-Fluoro-cyclopropanecarboxylic acid (1S,2S)-rel-2-Fluorocyclopropanecarboxylic acid Cyclopropanecarboxylic acid, 2-fluoro-, (1S-cis)- MFCD19237459 (cis)-2-fluorocyclopropanecarboxylic acid Cyclopropanecarboxylic acid, 2-fluoro-, (1S,2S)- (1S,2S)-2-fluorocyclopropanecarboxylicacid MFCD04972869 SCHEMBL1463730 AMY5475 DTXSID901262266 CFA19914 CS1221 AKOS006294678 |
| Molecular Formula | C4H5FO2 |
| Molecular Weight | 104.08 |
| InChI | InChI=1S/C4H5FO2/c5-3-1-2(3)4(6)7/h2-3H,1H2,(H,6,7)/t2-,3+/m1/s1 |
| InChI Key | HZQKMZGKYVDMCT-GBXIJSLDSA-N |
| Canonical SMILES | C1[C@H]([C@H]1F)C(=O)O |
Physical Data
| Appearance | White solid |
| Melting Point, °C | Solvent (Melting Point) |
| 55 – 56 | |
| 62 – 63 | toluene |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d6 | 400 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 100 |
| Chemical shifts | 1H | CDCl3 | |
| Chemical shifts | 19F | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| ATR (attenuated total reflectance), Bands | ||
| Bands | KBr | 3210 – 1190 cm**(-1) |
Route of Synthesis (ROS)
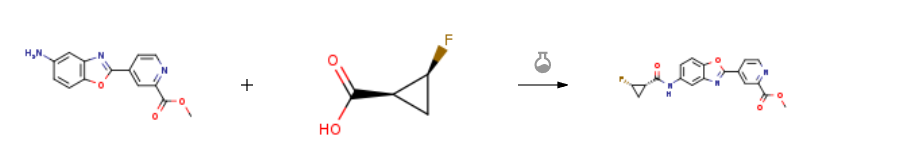
Route of Synthesis (ROS) of (1S,2S)-2-Fluorocyclopropanecarboxylic acid
| Conditions | Yield |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 10 – 30℃; for 5h; | 83.4% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; | 1.1 g |
| Experimental Procedure DMF (50 mL), (1S,2S)-2-fluorocyclopropanecarboxylic acid (425 mg, 4.1 mmol), HATU (2.1 g, 5.58 mmol) and DIEA (1.44 g, 11.16 mmol) were successively added to compound 4a (1.0 g, 3.71 mmol), and the mixture was stirred at room temperature for 5 h. The reaction was quenched by adding water, extracted 3 times with ethyl acetate and washed twice with saturated brine. The organic phase was dried and concentrated, and the residue was separated and purified by silica gel column chromatography (eluent: EA/PE=1/2) to obtain 117E (1.1 g, 83.4%). LC-MS (ESI): m/z=356.3 [M+H]+. |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P260, P261, P264, P271, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P319, P321, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store in room temperature for long time and dry place; in container tightly sealed; Protect from light. |
| HS Code | |
| Storage | Store in room temperature for long time and dry place; in container tightly sealed; Protect from light. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 104.081 |
| logP | 0.27 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.3 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| (1S,2S)-2-Fluorocyclopropanecarboxylic acid CAS#: 127199-14-8 and its derivatives can act as chiral catalysts in asymmetric synthesis reactions. They can guide the reaction selectively to produce chiral products, thereby enhancing the stereo-selectivity and efficiency of the reaction. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to [email protected] |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |