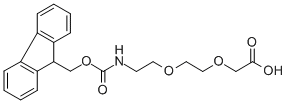
[2-[2-(Fmoc-amino)ethoxy]ethoxy]acetic acid CAS#: 166108-71-0; ChemWhat Code: 93432
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN114213283 | Method for preparing [2-[1-(Fmoc-amino) ethyoxyl] ethyoxyl] acetic acid by one-pot method | 2022 |
Physical Data
| Appearance | White powder |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | tetradeuteriomethanol | 300 |
| 1H | tetradeuteriomethanol | 300 | |
| Chemical shifts | 13C | tetradeuteriomethanol |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of [2-[2-(Fmoc-amino)ethoxy]ethoxy]acetic acid CAS# 166108-71-0
| Conditions | Yield |
| With anhydrous sodium carbonate In tetrahydrofuran at 15 – 20℃; for 3h; Experimental Procedure Compound A3 (0.2g, 1.23mmol), 2mL of tetrahydrofuran, 2mL of water and sodium carbonate (0.2g, 1.84mmol) were added to the reaction kettle, the reaction temperature was maintained at 15-20°C, and fluorene methoxycarbonyl succinimide was added. (abbreviated as Fmoc-Osu, 0.4 g, 1.23 mmol) to carry out acylation reaction, after 3 hours of reaction, spot plate to confirm the end point of the reaction, then add ethyl acetate to the reaction solution, adjust pH=2-3 with hydrochloric acid, stand for fractionation The organic phase was washed three times with saturated brine, dried over sodium sulfate, and concentrated to obtain Fmoc-AEEA (0.43 g, yield 91%, purity 99.8%).The purity of Fmoc-AEEA synthesized in this example is 99.8%, and the total yield is 88%×91%×93%×91%=67.78%. | 91% |
| With Sodium hydrogenocarbonate In ethanol; lithium hydroxide monohydrate for 6h; pH=9; Experimental Procedure Add sodium bicarbonate solid to adjust the product pH of step d to be about 9, then add 140mL of water, 140ml of ethanol, add 42.5g of Fmoc-osu in batches, react 6h after TLC monitoring, after the completion of the reaction; concentrate most of the ethanol under reduced pressure , the pH was adjusted to 2 with hydrochloric acid, crystal seeds were added for crystallization, the solid was precipitated and the crystallization continued for 2 h, filtered and dried to obtain 41.6 g of a white solid, namely [2-[1-(Fmoc-amino)ethoxy]ethoxy base]acetic acid.Result detection: the obtained [2-[1-(Fmoc-amino)ethoxy]ethoxy]acetic acid has a purity of 99.6% and a yield of 63.2%. | 63.2% |
| With potassium carbonate In lithium hydroxide monohydrate for 16h; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | At room temperature away from light |
| Storage | At room temperature away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 385.417 |
| logP | 2.159 |
| HBA | 7 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 94.09 |
| Rotatable Bond (RotB) | 12 |
| Matching Veber Rules | 1 |
| Use Pattern |
| [2-[2-(Fmoc-amino)ethoxy]ethoxy]acetic acid CAS#: 166108-71-0 is an intermediate of API Sermaglutide. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |


![Route of Synthesis (ROS) of [2-[2-(Fmoc-amino)ethoxy]ethoxy]acetic acid CAS# 166108-71-0](https://www.chemwhat.com/wp-content/uploads/2017/12/Route-of-Synthesis-ROS-of-2-2-Fmoc-aminoethoxyethoxyacetic-acid-CAS-166108-71-0.png)