2-Propanol CAS#: 67-63-0; ChemWhat Code: 881256
Identification
| Product Name | 2-Propanol |
| IUPAC Name | propan-2-ol |
| Molecular Structure | 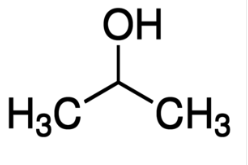 |
| CAS Registry Number | 67-63-0 |
| EINECS Number | 200-661-7 |
| MDL Number | MFCD00011674 |
| Beilstein Registry Number | 635639 |
| Synonyms | isopropyl alcohol, isopropanol, propan-2-ol, isopropylalcohol, 2-propanol, i-propanol |
| Molecular Formula | C3H8O |
| Molecular Weight | 60.095 |
| InChI | InChI=1S/C3H8O/c1-3(2)4/h3-4H,1-2H3 |
| InChI Key | KFZMGEQAYNKOFK-UHFFFAOYSA-N |
| Canonical SMILES | CC(C)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109265328 | A visible light driving green synthetic benzion bisether method (by machine translation) | 2019 |
| WO2019/27962 | IRON-CATALYZED CROSS-COUPLING OF METHHANOL WITH SECONDARY OR TERTIARY ALCOHOLS TO PRODUCE FORMATE ESTERS | 2019 |
| WO2019/25467 | SELECTIVE INHIBITORS OF NLRP3 INFLAMMASOME | 2019 |
| EP3489230 | CHEMICAL COMPOUND OF ISOCITRATE DEHYDROGENASE INHIBITOR, AND APPLICATION THEREOF | 2019 |
| JP2019/81800 | The heterocyclic compound for controlling arthropod method (by machine translation) | 2019 |
Physical Data
| Appearance | Colorless liquid |
| Solubility | water: soluble (completely) |
| Flash Point | 53 °F |
| Refractive index | n20/D 1.377(lit.) |
| Sensitivity | Air Sensitive & Hygroscopic |
| Melting Point, °C |
| -87.9 |
| -88 |
| -89.5 |
| -88.53 |
| -88.6 |
| -86 |
| -121 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 82.4 | |
| 355.42 | |
| 82.37 | 760.051 |
| 82.23 | 759.826 |
| 82.19 | 759.826 |
| 82.4 | 760.051 |
| 60.78 | 300.03 |
| 45 | 139.2 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.373 | 589 | 29.99 |
| 1.3754 | 589 | 25.04 |
| 1.3751 | 589 | 24.99 |
| 1.36586 | 589 | 44.99 |
| 1.37061 | 589 | 34.99 |
| 1.3776 | 589 | 20 |
| 1.375 | 589 | 25 |
| 1.3773 | 589 | 20 |
| 1.3708 – 1.3807 | 589 | 15-35 |
| 1.3779 | 589 | 293.2 |
| Density, g·cm-3 | Measurement Temperature, °C | Comment (Density) |
| 0.77661 | 29.99 | Liquid |
| 0.78074 | 24.99 | Liquid |
| 0.78125 | 25.04 | Liquid |
| 0.000781588 | 24.99 | Press = 1 atm; Liquid |
| 0.78099 | 24.99 | Press = 81500 Pa; Liquid |
| 0.785282 | 19.99 | Liquid |
| 0.78101 | 298.15 |
| Description (Adsorption (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Adsorption and desorption isotherms | 4C6H2O4S(2-)*4C2H3O2(1-)*Zr6O4(OH)4(12+) | |
| Adsorption isotherm | 29.84 | C18H12N6*C2H3N*Cu(1+)*BF4(1-) |
| Adsorption | 180 | 9.8 wt% zirconia supported on mesoporous silica |
| Adsorption and desorption isotherms | 30 | MIL-68(Al) |
| Further physical properties of the adsorbed molecule | 26.84 | magnesium doped aluminia |
| Adsorption | 20 | [Zn4O(3,5-dimethyl-4-carboxypyrazolato)3]n film on COOH-modified gold |
| Desorption | -173.15 – 226.85 | O-Ni(100) |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | benzene-d6 | 24.84 | pyridine |
| UV/VIS spectrum of the complex | hexane | C46H15F15N4O2(2-)*Zn(2+) | |
| Association with compound | hexane | 24.84 | acetonitrile |
| Association with compound | hexane | diethyl ether | |
| Association with compound | tetrachloromethane | 24.84 | pyridine |
| Association with compound | benzene | 24.84 | diethyl ether |
| Association with compound | 1,2-dichloro-ethane | 24.84 | dimethyl sulfoxide |
| Association with compound | tetrachloromethane | 24.84 | tetrahydrofuran |
| Stability constant of the complex with … | CCl4 | methacrylic acid methyl ester |
| Temperature (Azeotropes (MCS)), °C | Pressure (Azeotropes (MCS)), Torr | Azeotropes |
| 72.79 | 759.826 | 1,3-DIOXOLANE |
| 79.85 | 706.557 | diethyl acetal |
| 70.84 | 759.811 | 2,2,4-trimethylpentane, water |
| 59.85 | 549.794 | Ethyl tert-butyl ether |
| 68.53 | 759.811 | cyclohexane, ethyl acetate, butanone |
| 103.67 | 2265.18 | cyclohexane, ethyl acetate, butanone |
| 77 | 759.8 | methyl cyclohexane |
| 21 | 102.08 | benzene, cyclohexane |
| 5 | 13 | propyl cyanide |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
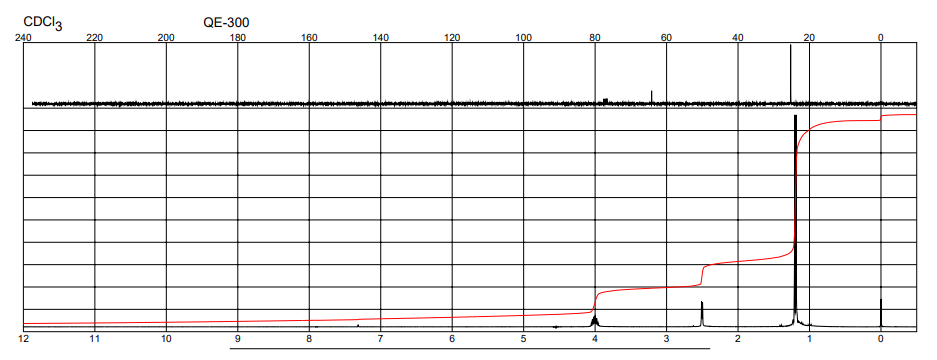
| NMR with shift reagents, Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts, Spectrum | 1H | water-d2 | 24.84 | 500 |
| Chemical shifts | 1H | chloroform-d1 | 300 | |
| Spectrum | 13C | |||
| Chemical shifts | 17O | various solvent(s) | 57 | 67.8 |
| Spectrum | 17O | various solvent(s) | 57 | 67.8 |
| Spectrum | 1H | benzene-d6 | 300 | |
| CIDNP | 1H | |||
| Chemical shifts | 13C | D2O | 24 | |
| Chemical shifts | 13C | CD3CN | 24 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
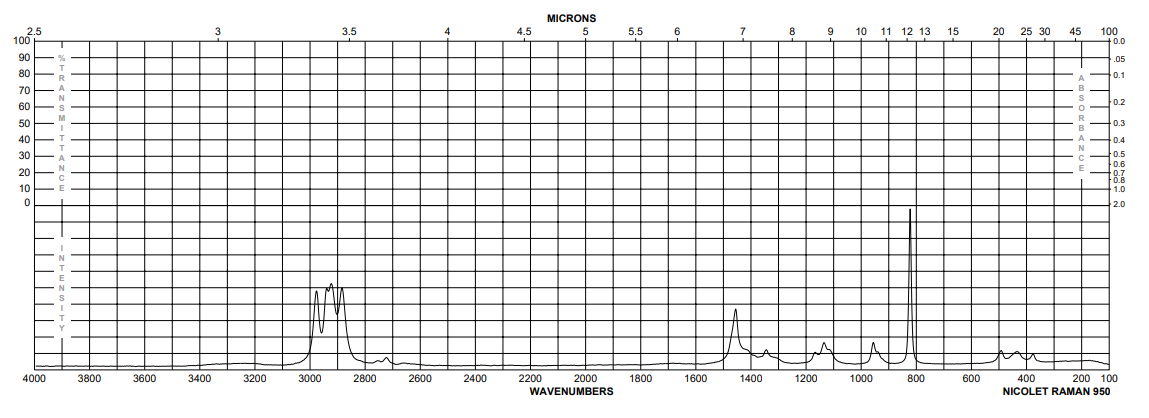
| Spectrum | toluene | ||
| Bands | heptane | 25 | |
| Near IR spectrum | temperature dependence | ||
| Bands | diethyl ether | 32 | |
| Bands | CH2Cl2 | 12 | |
| Spectrum | cyclohexane | concentration dependence | |
| Spectrum | gas | ambient temperature | |
| Spectrum | neat (no solvent) | -195.2 | 3000 – 750 cm**(-1) |
| Bands | neat (no solvent) | -2.6 – 58.7 | 3343 – 3318 cm**(-1) |
| Spectrum | solid | -258.2 | 3400 – 3000 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | gas | 1000 – 500 nm |
| Spectrum | methanol | 232.56 – 400 nm |
| Spectrum | 2,2,4-trimethyl-pentane | 181.82 – 400 nm |
| Spectrum | 181.82 – 400 nm, fluessig. | |
| Spectrum | Dampf und Fluessigkeit. | |
| Spectrum | elektrische Absorbtion. | |
| Absorption maxima | iodine | |
| UV/VIS |
| Description (Raman Spectroscopy) | Solvent (Raman Spectroscopy) | Comment (Raman Spectroscopy) |
| Spectrum | H2O | ambient temperature. Object(s) of Study: concentration dependence |
| Bands | ambient temperature | |
| Raman intensities | ||
| Raman | ||
| Spectrum | fluessig |
Route of Synthesis (ROS)
| Conditions | Yield |
| With trichlorophosphate In acetone at 10 – 30℃; under 10 Torr; for 4h; | 97% |
| With phosphorous; tetraethylammonium iodide In water; acetonitrile at 50℃; Electrolysis; | 80% |
| With phosphorous; 10H-phenothiazine; N,N,N,N-tetraethylammonium tetrafluoroborate In acetonitrile at 53℃; electrolysis; | 60% |
| With pyridine; trichlorophosphate; benzene zuerst unter Kuehlung mit Eis-NaCl, dann bei 80grad; |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H225: Highly Flammable liquid and vapor [Danger Flammable liquids] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H336: May cause drowsiness or dizziness [Warning Specific target organ toxicity, single exposure; Narcotic effects][Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 3; Packaging Group: II; UN Number: 1219 |
| Under the room temperature and away from light | |
| HS Code | 290512 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 60.0959 |
| logP | 0.384 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 20.23 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Effect |
| 6.72 | EC50 | 0.19 | µM | antiproliferative agent | ||
| 5.04 | IC50 | = | 9.2 | µM | Cytochrome P450 2E1 [human]:Wild | |
| 4.28 | activity rate | 130 | % | glucuronoarabinoxylan endo-1,4-beta-xylanase [Geobacillus stearothermophilus]:Wild | ||
| 2.38 | EC | 250 | mg/L | Behavioural Symptoms | ||
| 2.17 | Kd (dissociation constant) | 6.74 | mM | Cytochrome P450 3A4:Wild | ||
| 1.48 | MIC | 2000 | μg/ml | antifungal agent | ||
| 1.18 | Ki (inhibition constant) | 4000 | μg/ml | antifungal agent | ||
| 1 | Activity(relative activity) | Not active | Alcohol Dehydrogenase 6 (Class V) [Saccharomyces cerevisiae]:Wild | |||
| 1 | activity rate | 7 | % | glucuronoarabinoxylan endo-1,4-beta-xylanase [Geobacillus stearothermophilus]:Wild |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Biological Species | Route of administration | Dose | Effect |
| 2.92 | LD50 | 72.6 | mg/L | rat | inhalational administration | Toxic | |
| 2.08 | NOAEC | 500 | ppm | inhalational administration | 500ppm | Behavioural Symptoms | |
| LD50 | 5000 | mg/kg | |||||
| rat | oral administration | Toxic | |||||
| activation spikes(of cPA receptor) | 25.3 | -1 | Aedes aegypti | ||||
| biting rate | 100 | % | human | topical administration | 5% | repellent agent | |
| repellency percentage | 4 | % | Rhipicephalus sanguineus | repellent agent |
| Metabolism |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Dose |
| 4.48 | Ki (inhibition constant) | 33 | µM | Cytochrome P450 2E1 [rat]:Wild | 0.0100000 mM | |
| 2.87 | inhibition rate | 55 | % | Multidrug resistance protein 1 [human]:Wild | 100 µg/mL | |
| Fold-increase | 1.2 | fold | Cytochrome P450 2C9 [human]:Wild | 1% | ||
| IC50 | <= | 40 | % (v/v) | Alcohol dehydrogenase:Wild | ||
| pIC50(p-hydroxylation of aniline) | -0.47 | Cytochrome P450 (gene cypA):Wild | ||||
| t1/2 el | 3 | hour | ||||
| log K | 2.99 | |||||
| log BB(Blood level) | -0.15 |
| Pharmacokinetic |
| Quantitative Results |
| 1 of 10 | Biological material | human |
| Assay Description | Unchanged isopropyl alcohol may be excreted in the urine | |
| Results | Urinary excretion ND | |
| Measurement | Urinary excretion |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Effect |
| 1.26 | pIC50 | 1.26 | % | |
| EC20 | 16400 | mg/L | Toxic | |
| MIC | 50 | mL/L | antifungal agent | |
| TDLo(Lowest toxic dose) | 13000 | mg/kg | ||
| LDLo(Lowest lethal dose) | 5272 | mg/kg | ||
| Toxicity | -1.92 | |||
| pLC50 | -4.83 | Death | ||
| pGI50(Toxicity) | -1.8819 |
| Qualitative Results | ||
| 1 of 8 | Assay Description | Toxicity of compound was determined using Tadpole narcosis test |
| Results | log C not calculated | |
| Reference | Wilson; Famini[Journal of Medicinal Chemistry, 1991, vol. 34, # 5, p. 1668 – 1674] | |
| Measurement | log C | |
| 2 of 8 | Biological material | guppy |
| Assay Description | Konemann’s industrial pollutants was determined on Poecilia reticulata (Toxicity test) | |
| Reference | Wilson; Famini[Journal of Medicinal Chemistry, 1991, vol. 34, # 5, p. 1668 – 1674] | |
| 3 of 8 | Biological material | rat |
| Assay Description | Lowest observable effect level for maternal toxicity in rat | |
| Reference | No author[Toxicology., Edited by Hans Marquardt, Siegfried G. Schafer, Roger McClellan and Frank Welsch, Published by Academic Press (US), 1999] | |
| 4 of 8 | Biological material | rat |
| Assay Description | Lowest observable effect level for fetal toxicity in rat | |
| Reference | No author[Toxicology., Edited by Hans Marquardt, Siegfried G. Schafer, Roger McClellan and Frank Welsch, Published by Academic Press (US), 1999] | |
| 5 of 8 | Biological material | guppy |
| Assay Description | Aquatic toxicity of the compound in Poecilia reticulata | |
| Reference | Katritzky; Tatham; Maran[Journal of Chemical Information and Computer Sciences, 2001, vol. 41, # 5, p. 1162 – 1176] | |
| 6 of 8 | Biological material | rat |
| Assay Description | After inhalation rat shows irration of mucosa, dose and time-dependent CNS depression;Inhalation;8 hour | |
| Reference | No author[Toxicology., Edited by Hans Marquardt, Siegfried G. Schafer, Roger McClellan and Frank Welsch, Published by Academic Press (US), 1999] | |
| 7 of 8 | Effect | Cytotoxic |
| Assay Description | Effect : MTT conversion Target : rat hepatoma Fa32 cells Bioassay : MEIC reference comp. (MEIC = Multicentre Evaluation of in Vitro Cytotoxicity); MTT50 = conc. required to reduce MTT conversion by 50 percent as compared to control cells in vitro; 64-well microplates; complete medium; 30 min incub.; MTT assay | |
| Results | MTT50 765 mmol/l | |
| Reference | Dierickx, Paul J.[Toxicology, 2000, vol. 150, # 1-3, p. 159 – 169] | |
| 8 of 8 | Effect | antiviral agent |
| Assay Description | Target : adenovirus type 8 ATCC VR-1085AS/RB Bioassay : ATCC: American Type Culture Collection virus incubated with title comp. for 1 or 5 min at 22 deg C; mixture neutralized and virus titrated in cell cultures (A549 cells); cytopathic effects observed; efficacy criteria of a 3-log;10 reduction in titer of virus infectivity used | |
| Reference | Rutala, William A.; Peacock, Jeffrey E.; Gergen, Maria F.; Sobsey, Mark D.; Weber, David J.[Antimicrobial Agents and Chemotherapy, 2006, vol. 50, # 4, p. 1419 – 1424] |
| Use Pattern |
| 2-Propanol CAS# 67-63-0 is used as an extraction solvent and carrier solvent for chemical reagents and chromatography reagents. |
| 2-Propanol CAS# 67-63-0 used for beet sugar, candy, nutritional supplement tablets, hop extract, lemon oil, spice oleoresin, yeast and other processing. |
| 2-Propanol CAS# 67-63-0 has a wide range of uses as organic raw materials and solvents. As chemical raw materials, it can produce acetone, hydrogen peroxide, methyl isobutyl ketone, diisobutyl ketone, isopropylamine, isopropyl ether, isopropyl alcohol ether, isopropyl chloride, and fatty acid isopropyl ester and chlorine Fatty acid isopropyl ester and so on. |
| 2-Propanol CAS# 67-63-0 is used in the production of coatings, inks, extractants, aerosols, etc. |
| Isopropanol is an important intermediate for pesticide production |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |