2-(Trimethylsilyl)ethoxymethyl chloride CAS#: 76513-69-4; ChemWhat Code: 1489906
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN107226814 | Preparation method for intermediate of Baricitinib | 2017 |
| US2012/22581 | SUBSTITUTED FUSED PYRIMIDINE COMPOUNDS, ITS PREPARATION AND USES THEREOF | 2012 |
Physical Data
| Appearance | Colourless to light yellow liquid |
| Melting Point, °C |
| 57 – 59 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 57 – 59 | 8 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.05 | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | 300 | |
| Spectrum | 13C | ||
| Chemical shifts | 13C | acetone-d6 | 68 |
| Chemical shifts | 1H | acetone-d6 | 300 |
| 1H | acetoacetone-d6 | 300 | |
| Chemical shifts | 1H | CDCl3 | |
| Chemical shifts | 13C | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
Route of Synthesis (ROS)
| Conditions | Yield |
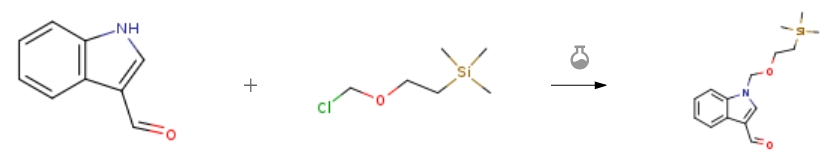
| Stage #1: Indole-3-carboxaldehyde With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In N,N-dimethyl-formamide for 2h; | 98% |
| Stage #1: Indole-3-carboxaldehyde With sodium hydride In N,N-dimethyl-formamide at 0 – 20℃; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride at 0 – 20℃; Experimental Procedure 2.4. Typical procedure for the preparation of 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-3-carbaldehydes: synthesis of the 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-3-carbaldehyde To a stirred suspension of NaH (331 mg, 8.27 mmol, 1.2 equiv.) in DMF anhydrous (5 mL) was added dropwise a solution of 1H-indole-3-carbaldehyde (1.0 g, 6.89 mmol, 1.0 equiv.) dissolved in anhydrous DMF (10.0 mL) at 0°C under argon. The mixture was stirred for 1 hour, warmed to room temperature and stirred until the salt was formed. Then, reaction mixture was cooled to 0°C before adding dropwise SEMCl (1.40 mL, 7.58mmol, 1.1 equiv.), warmed to room temperature and stirred for 10 minutes. After the consumption of the substrate (TLC, n-hexane-EtOAc, 80:20), the reaction was diluted with Et2O, washed with a solution of KHSO4 (10% w/w), a saturated solution of NaHCO3, and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25-40 μm), eluting with 85/15 (v/v) n-hexane/AcOEt mixture (Rf = 0.22) to obtain 1.85 g (97% yield) of 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-3-carbaldehyde. | 97% |
| With sodium hydride Substitution; | 85% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H226 (100%): Flammable liquid and vapor [Warning Flammable liquids] H314 (97.92%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318 (10.42%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P260, P264, P264+P265, P280, P301+P330+P331, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P317, P321, P363, P370+P378, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 166.723 |
| logP | 2.264 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 9.23 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2-(Trimethylsilyl)ethoxymethyl chloride CAS 76513-69-4 (SEM-Cl) is an organosilicon compound commonly used as a protecting group in organic synthesis. SEM-Cl is frequently used as a protecting group in organic synthesis, particularly for protecting hydroxyl (-OH) and amino (-NH2) groups. The use of protecting groups can prevent these functional groups from undergoing unwanted reactions during the synthesis process, thereby increasing the selectivity and efficiency of the synthesis. In the synthesis of complex organic molecules, SEM-Cl can be used to temporarily protect certain functional groups. Once other parts of the synthesis are completed, the protecting group can be removed under specific conditions to restore the original functional group. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |