2,2-DIMETHYL-3-PENTANONE CAS#: 564-04-5; ChemWhat Code: 798923
Identification
| Product Name | 2,2-DIMETHYL-3-PENTANONE |
| IUPAC Name | 2,2-dimethylpentan-3-one |
| Molecular Structure | 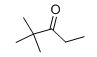 |
| CAS Registry Number | 564-04-5 |
| EINECS Number | No data available |
| MDL Number | MFCD00039918 |
| Beilstein Registry Number | No data available |
| Synonyms | 2,2-dimethyl-3-pentanone, 2,2-dimethylpentan-3-one, tert-butyl ethyl ketone, 2,2-dimethylpent-3-one, t-butyl ethyl ketone, 4,4-dimethyl-3-pentanone, ethyl tert-butyl ketone CAS Number 564-04-5 CAS NO 564-04-5 |
| Molecular Formula | C7H14O |
| Molecular Weight | 114.186 |
| InChI | InChI=1S/C7H14O/c1-5-6(8)7(2,3)4/h5H2,1-4H3 |
| InChI Key | VLNUTKMHYLQCQB-UHFFFAOYSA-N |
| Canonical SMILES | CCC(=O)C(C)(C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US4240983 | Monohalogenated ketones | 1980 |
Physical Data
| Appearance | No data available |
| Solubility | It is soluble in water as well as soluble in alcohol, benzene. |
| Refractive index | 1.4028 |
| Melting Point, °C | Solvent (Melting Point) |
| 123 | |
| 122 – 127 | |
| -49 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.4037 | 589 | 20 |
| 1.405 | 589 | 20 |
| 1.4028 | 589 | 25 |
| 1.403 | 589 | 25 |
| 1.4109 | 589 | 20 |
| 1.4057 | 589 | 20 |
| 1.4049 | 589 | 20 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 118 – 125 | 3 |
| 125 | 759.8 |
| 123 – 126 | 720 |
| 79 | 157 |
| 68 – 69 | 12 |
| 124 – 125 | 680 |
| 124.5 | 730 |
| 125 – 126 | 769 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.811 | 4 | 20 |
| 0.8098 | 4 | 20 |
| 0.8017 | 25 | 25 |
| 0.8258 | 0 | 0 |
| 0.8106 | 0 | 20 |
| 0.81 | 0 | 21 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | CCl4 | 28 | 2.6-dimethylphenol |
| Association with compound | CCl4 | 28 | 2,5-Dimethylphenol |
| Association with compound | CCl4 | 28 | phenol |
| Association with compound | CCl4 | 28 | 4-chloro-phenol |
| Association with compound | CCl4 | 28 | 3,5-dichlorophenol |
| Association with compound | CCl4 | 28 | 2-monochlorophenol |
| Association with compound | CCl4 | 28 | 2-hydroxybromobenzene |
| Association with compound | CCl4 | 28 | 2,4,6-Trichlorophenol |
| Association with compound | CCl4 | 28 | 2-Iodophenol |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 101 |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 |
| Chemical shifts | 1H | CDCl3 | 300 |
| 1H | CDCl3 | 300 | |
| Chemical shifts | 13C | CDCl3 | 75 |
| Chemical shifts | 1H | ||
| 13C | benzene-d6 | ||
| Spin-spin coupling constants | benzene-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Mid IR (MIR), Bands | film | |
| Bands | neat (no solvent) | 1710 cm**(-1) |
| Bands | hexane | 1715 cm**(-1) |
| Bands | CCl4 | 1711 cm**(-1) |
| Bands | tetrahydrofuran | 1715 cm**(-1) |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| electron impact (EI), gas chromatography mass spectrometry (GCMS), spectrum | |
| chemical ionization (CI), spectrum | |
| fragmentation pattern, spectrum | MIKE (mass ion kinetic energy) |
| metastable ions, collisional activation | |
| spectrum | |
| fragmentation pattern | collisional activation |
| negative ion spectroscopy | |
| spectrum | collisional activation |
| metastable ions | |
| spectrum, electron impact (EI) | charge exchange with positive ions |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| UV/VIS | |||
| Spectrum | diethyl ether | 260 – 310 nm | |
| Absorption maxima | hexane | 232, 290 | |
| Absorption maxima | ethanol | 284 | |
| Absorption maxima | H2O | 279 |
Route of Synthesis (ROS)
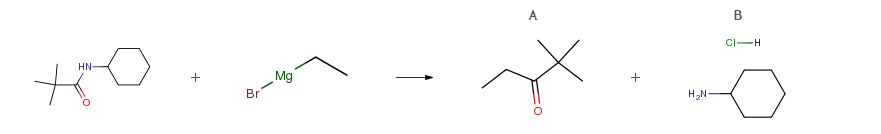
| Conditions | Yield |
| Stage #1: N-cyclohexyl-2,2-dimethylpropanamide With 2-fluoropyridine; trifluoromethylsulfonic anhydride In dichloromethane at 0℃; for 0.5h; Stage #2: ethylmagnesium bromide With cerium(III) chloride In tetrahydrofuran; dichloromethane at -78℃; for 2h; Stage #3: With hydrogenchloride In ethyl acetate Experimental Procedure General procedure: Tf2O (185μL, 1.1mmol) was added dropwise to a cooled (0°C) solution of amide (1.0mmol) and 2-fluoropyridine (103μL, 1.2mmol) in dichloromethane (4mL). After stirring at 0°C for 30min, the mixture was cannulated to a freshly prepared organocerium reagent/complex (3.0mmol) in THF (15mL) at −78°C and stirred for 2h. Aqueous HCl solution (3mol/L, 5mL) was added to quench the reaction and the mixture was allowed to warm to r.t. and stirred for 2h. Ammonium hydroxide solution (25percent, 5mL) was then added to the mixture. The organic layer was separated and the aqueous phase was extracted with diethyl ether (3× 10mL). The combined organic layers were washed with brine (3× 3mL) and concentrated under reduced pressure to about 1/3 volume. The residual organic phase was then extracted with aqueous HCl solution (3mol/L, 3× 5mL). The separated organic phase was washed with brine (5mL), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure, and the residue was purified by flash column chromatography on silica gel to afford ketone. The aqueous phases were combined, washed with diethyl ether (5mL), basified with an ammonium hydroxide solution (25percent, 5mL) and back-extracted with diethyl ether (5× 20mL). The ether layers were combined, washed with brine (5mL), dried over anhydrous MgSO4, filtered, acidified with a solution of HCl in ethyl acetate (3mol/L, 5mL) and concentrated under reduced pressure to afford the desired amine hydrochloride salt. | A n/a B 74% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H319 (95.83%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P280, P305+P351+P338, and P337+P313 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 291419 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data available |
| Market Price | USD |
| Use Pattern |
| 2,2-DIMETHYL-3-PENTANONE CAS#: 564-04-5 is commonly used as an intermediate in the synthesis of perfumes. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

