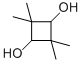
2,2,4,4-TETRAMETHYL-1,3-CYCLOBUTANEDIOL CAS#: 3010-96-6; ChemWhat Code: 356772
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2019/11046 | METHOD FOR PREPARING ETHERS OF CYCLOALIPHATIC OR ARALIPHATIC DIOLS | 2019 |
| US2017/334815 | CATALYST AND METHOD FOR HYDROGENATION OF 1,3-CYCLOBUTANEDIKETONE COMPOUND | 2017 |
| WO2012/170264 | BISPHENOL COMPOUNDS AND METHODS OF MAKING | 2012 |
| US2002/141952 | PYRROLOTRIAZINE DERIVATIVES AS KINASE INHIBITOR | 2002 |
Physical Data
| Appearance | White crystalline powder |
| Melting Point, °C |
| 128 – 150 |
| 130 – 133 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts, Spectrum | 1H | ||
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 20 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 20 |
Route of Synthesis (ROS)
| Conditions | Yield |
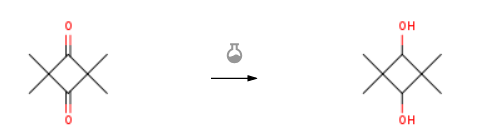
| With platinum on carbon; hydrogen In ethanol at 22℃; under 4500.45 Torr; for 2h; Autoclave; Experimental Procedure Add 320 ml of ethanol to the dry autoclave, and then add 39.25 g of TMCB (0.28 mol),Add 10g of platinum on carbon and start to feed hydrogen to keep the internal pressure of the reactor at 0.6 MPa.The internal temperature is 22°C, and the reaction is two hours. After the reaction is over, the reaction solution is poured into a 500 ml beaker,After filtering off the platinum charcoal (recyclable for use), the mother liquor is rotated in a water bath at 40°C to remove the solvent.Add 80 ml petroleum ether to disperse, collect the solid product after filtration,Obtained 37.5 g (0.26 mol, dry weight of 36 g) CBDO product, white in appearance, 99.2% content, and 91.7% reaction yield. | 91.7% |
| With lithium aluminium tetrahydride | |
| With hydrogen at 180℃; under 30003 Torr; Reagent/catalyst; Experimental Procedure 2, 2,4,4-tetramethyl-1,3-cyclobutanedione was hydrogenated using a known fixed bed hydrotreating apparatus,Preparation 2, 2,4,4-tetramethyl-1,3-cyclobutanediol, catalyst selection Example 3. Catalyst, catalyst loading: 3 ml, reaction pressure: 4. OmPa, reaction temperature: 180 ° C, raw material: 8.8 g 2, 2,4,4-tetramethyl-1,3-cyclobutanedione / 100 ml 1,4-cyclohexanedicarboxylic acid dimethyl ester, feed rate: 9 Ml / hour, Η2: 1.4 liters / min, the results are listed in Table 1. |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H228 (25.9%): Flammable solid [Danger Flammable solids] H302 (73.4%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (26.6%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (26.6%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (25.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P210, P240, P241, P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Preserve in a well-closed container and keep in cool, dry place |
| Storage | Preserve in a well-closed container and keep in cool, dry place |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 144.214 |
| logP | 0.756 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.46 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2,2,4,4-Tetramethyl-1,3-cyclobutanediol (CBDO) is a symmetrical, rigid diol monomer widely used in high-performance copolyesters (e.g., PETG, PCTG), thermoplastic polyurethanes (TPU), optical materials, and advanced coatings and adhesives. As a BPA-free alternative, CBDO imparts excellent transparency, heat resistance, dimensional stability, and optical clarity, making it suitable for applications in packaging, healthcare, electronics, and aerospace. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Limited | <a href="http://www.watsonnoke.com/2244-tetramethyl-13-cyclobutanediol-cas-3010-96-6/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |