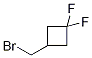
3-(Bromomethyl)-1,1-difluorocyclobutane CAS#: 1252934-30-7; ChemWhat Code: 1078901
Identification
Physical Data
| Appearance | Colorless liquid |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6, tetrachloromethane | 400 |
Route of Synthesis (ROS)
| Conditions | Yield |
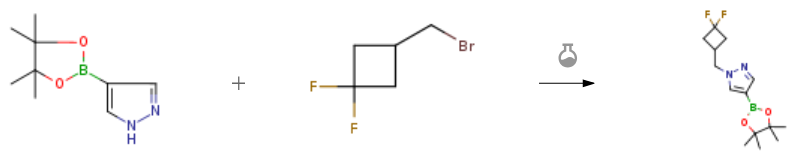
| With caesium carbonate In N,N-dimethyl-formamide at 0 – 20℃; for 18h; Experimental Procedure Step 1: l-[(3,3-difluorocyclobutyl)methyl]-4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)pyrazole A solution of 3-(bromomethyl)-l,l-difluorocyclobutane (195 mg, 1.05 mmol) in DMF (0.6 mL) was added to a stirred suspension of 4-pyrazoleboronic acid pinacol ester (200 mg, 1.03 mmol) and cesium carbonate (537 mg, 1.65 mmol) in DMF (1.4 ml) at 0 °C. The reaction mixture was stirred at rt for 18h, then filtered, washing with EtOAc. The filtrate was washed with brine (2x), dried over NaaSCfi, filtered and concentrated under reduced pressure to afford l-[(3,3-difluorocyclobutyl)methyl]-4-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)pyrazole (270 mg, 0.9 mmol, 88 % yield) as colorless oil. LC/MS (ESI+) m/z = 299.1 [M+H]+. | 88% |
| With caesium carbonate In N,N-dimethyl-formamide at 0 – 20℃; for 18h; Experimental Procedure 1 Step 1: 1-[(3,3-difluorocyclobutyl)methyl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole. A solution of 3-(bromomethyl)-1,1-difluorocyclobutane (195 mg, 1.05 mmol) in DMF (0.6 mL) was added to a stirred suspension of 4-pyrazoleboronic acid pinacol ester (200 mg, 1.03 mmol) and cesium carbonate (537 mg, 1.65 mmol) in DMF (1.4 ml) at 0°C. The reaction mixture was stirred at rt for 18 h, then filtered, washing with EtOAc. The filtrate was washed with brine (2x), dried over Na2SO4, filtered and concentrated under reduced pressure to afford 1-[(3,3-difluorocyclobutyl)methyl]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole as a colorless oil. (270 mg, 0.9 mmol, 88% yield). LC/MS (ESI+) m/z = 299.1 [M+H]+. | 88% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H226 (100%): Flammable liquid and vapor [Warning Flammable liquids] H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| HS Code | |
| Storage | Store at 2°C ~ 8°C for long time, in container tightly sealed; Protect from light. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 185.011 |
| logP | 2.932 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| The main use of 3-(bromomethyl)-1,1-difluorocyclobutane is as a compound for research purposes. It may be used in organic synthesis research, particularly in the development of new synthetic pathways or as an intermediate for the preparation of other chemical substances. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |