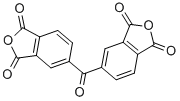
3,3′,4,4′-Benzophenonetetracarboxylic dianhydride CAS#: 2421-28-5; ChemWhat Code: 28100
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2024/336599 | PHOTOSENSITIVE POLYAMIC ACID AND DIAZIRINE COMPOSITIONS | 2024 |
| CN111217839 | 2, 6-disubstituted pyrimidine aromatic anhydride as well as synthesis method and application thereof | 2020 |
| CN108503647 | Benzocyclobutene end-capped imide monomer as well as preparation method and curing method thereof | 2018 |
| JP2017/160131 | (METH) ACRYL IMIDE COMPOUND AND INK PREPARED THEREWITH | 2017 |
| CN104058993 | Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element | 2017 |
| JP2017/202987 | (METH) ACRYL IMIDE COMPOUND AND INK PREPARED THEREWITH | 2017 |
| JP2017/202988 | (METH) ACRYL IMIDE COMPOUND AND INK PREPARED THEREWITH | 2017 |
Physical Data
| Appearance | White crystalline powder |
| Melting Point, °C |
| 228.4 |
| 223 – 224 |
| 220 |
| 308 – 309 |
| 241 |
| 221 |
| 225 |
| Description (Association (MCS)) |
| Spectrum of the complex |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Spectrum | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 13C | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 |
| Chemical shifts | 1H | chloroform-d1 | 600 |
| Spectrum | 1H | ||
| NMR |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | |
| Bands, Spectrum | |
| ATR (attenuated total reflectance), Bands, Spectrum | |
| Bands | potassium bromide |
| Bands | KBr |
| IR |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | acetonitrile | 300, 580 |
| Spectrum | 297 | |
| Spectrum | ||
| UV/VIS |
Route of Synthesis (ROS)
| Conditions | Yield |
| With 2,6-bis[(2,2,6,6-tetramethylpiperidin-1-yl)methyl]phenylboronic acid In Butyronitrile for 12h; Reflux; | 98% |
| With polyphosphoric acid In toluene at 100 – 110℃; for 6.5h; Reagent/catalyst; Temperature; Experimental Procedure 1.3; 2.3; 3.3 Step (3): add polyphosphoric acid (32g) and toluene (72g) into a 250mL three-necked flask,The intermediate (II) obtained in the above step is stirred under stirring(18.0g, 0.052mol) was added to the reaction solution,The internal temperature was adjusted to 100 to 110°C and stirred for 6.5 hours.Slowly cool down to 20 ~ 30 , filter,The filter cake was rinsed with toluene (30 g) and dried under vacuum at 80°C for 13 hours.14.9 g of yellow final product was obtained, with a purity of 98.9%,Step (3) Molar yield: 92.1%. The three-step total molar yield: 63.0%. | 92.1% |
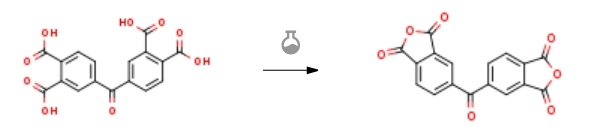
| at 220℃; for 1h; Temperature; 1.S3; 1.S2; 1; 2.S3; 3.S3; 4.S3; 5.S3; 6.S3; 7.S3; 8.S3 S3, the 3,3′,4,4′-benzophenone tetracarboxylic acid is placed in a high temperature vacuum sintering furnace and heated to 220°C for melting and dehydration for 1h,get white solid3,3′,4,4′-benzophenone tetracarboxylic dianhydride, the calculated yield was 89.6%, and the purity (high performance liquid chromatography) was 99.2%. | 89.6% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264+P265, P271, P280, P304+P340, P305+P351+P338, P319, P337+P317, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature and away from light |
| HS Code | |
| Storage | Under room temperature and away from light |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 322.23 |
| logP | 2.578 |
| HBA | 7 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 103.81 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Synthesis of ketone anhydride, polyimides, used in foams, paints, films, fibers, composites, BTDA containing polyimides have excellent thermal oxidative stability, strength, solvent resistance, flexibility. As an excellent epoxy curing agent, the resin has a high temperature stability, excellent electrical properties, chemical resistance, solvent resistance with BTDA. In the polyester resin, ester formulations, urethane resin, alkyd resin, polybenzimidazole pyrrolidone and other formulations, one BTDA anhydride can be added excellent high temperature and oxidative stability. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |