| Conditions | Yield |
With copper(II) nitrate monohydrate; acetic anhydride; acetic acid at 100℃; for 0.5h;
Experimental Procedure
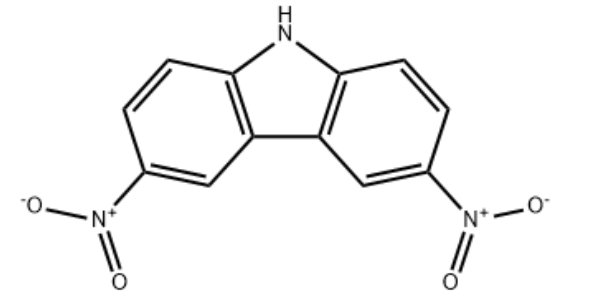
3.1 2.3.1. Synthesis of 3,6-Dinitrocarbazole (1)
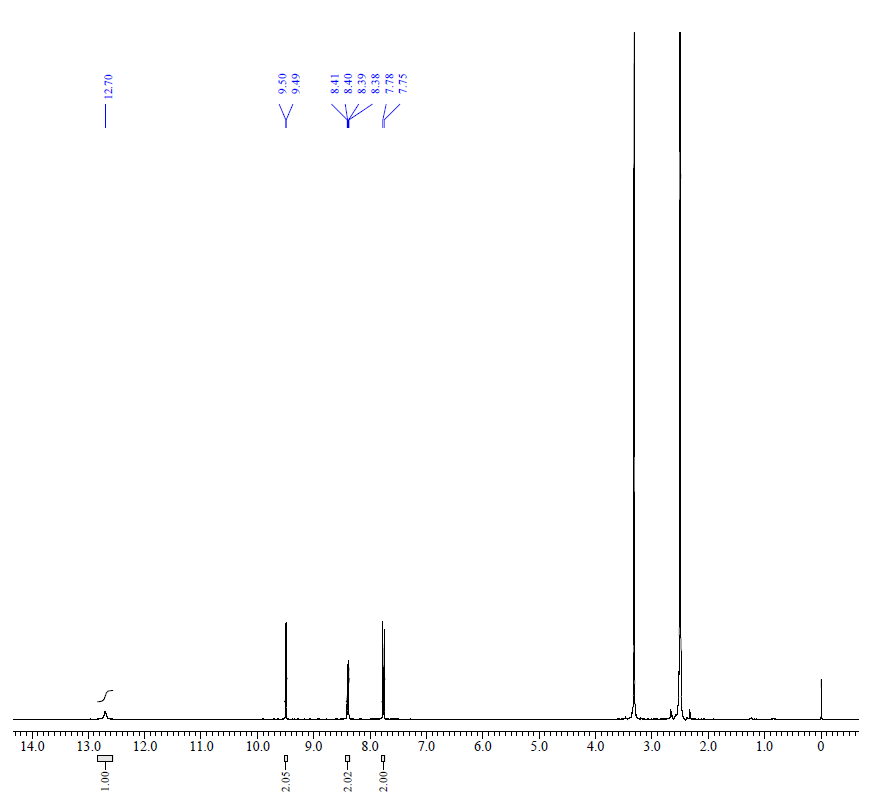
Cu(NO3)2·H2O (5.23 g, 22 mmol) was added into a mixture of acetic acid (10 mL) and acetic anhydride (25 mL) at room temperature. The mixture was stirred for 10-20 min, and to this solution was then added carbazole (3.00 g, 18 mmol) slowly in portions over 5 min [32]. Heat was generated during the addition (temperature rose to around 100 °C), and an additional 10 mL of acetic acid was added. The mixture was stirred at this temperature for 30 min and then poured into distilled water (200 mL). The precipitate was collected by filtration, washed with water (100 mL × 3). The still wet product was then dissolved into a potassium alcoholic aqueous solution (KOH 25 g, water 250 mL, and ethanol 250 mL). The solution turned red. After being stirred for 30 min, the solution was filtrated and the filtrate was acidified with concentrated hydrochloric acid. The yellow precipitate was then collected by filtration, washed with water and dried at 100 °C under vacuum to give yellow solid 3.94 g (85%). Mp: 242-243 °C; FT-IR (KBr, cm-1): 3091 (N-H stretching), 1508, 1328 (-NO2 asymmetric and symmetric stretching), 860 (C-N); 1H NMR (400 MHz, DMSO-d6) δ 11.10 (s, 1 H, NH), 9.34 (d, 2 H, J = 2.3 Hz), 8.40 (dd, 2 H, J = 2.3 Hz, J = 8.9 Hz), 7.78 (d, 2 H, J = 8.9 Hz). | 85% |
With copper(II) nitrate trihydrate; acetic anhydride; acetic acid at 100℃; for 0.5h;
Experimental Procedure
1.1 Synthesis of (1) 3, 6-dinitro-carbazole
A mixture of Cu (N03) 2 · 3H20 (5. 23g, 22. OOmmol) was added to a 100mL three-necked flask, and thenWere added 10mL of acetic acid and 20mL of acetic anhydride, stirred at room temperature for 10min to dissolve the solid; weighed carbazole (3. 00g,18. OOmmol) batch was slowly added to the solution to produce a lot of heat during the addition, the temperature rose to about 100 ° C, supplementedAdd 10mL of acetic acid, the reaction 30min. Completion of the reaction, the reaction mixture was poured into 200mL of distilled water, stirred, filtered, washed with distilled waterWashed until the eluate is neutral; filtration cake obtained is dissolved in a first alkali (manufactured by 25g K0H, 250mL of distilled water, 250mLDubbed ethanol), stirred for 30min, filtered to give a brown filtrate with concentrated hydrochloric acid (36% mass fraction of concentrated hydrochloric acid) was acidified filtrateTo a large number of yellow solid was precipitated, the precipitate was filtered, washed with water several times, and dried to give a yellow solid 3. 94g, that is, 3, 6-Nitro carbazole, 85% yield. | 85% |
With copper nitrate hemi(pentahydrate); acetic anhydride; acetic acid at 15 – 90℃; for 1.16667h;
Experimental Procedure
4.1.1. 3,6-Dinitro-9H-carbazole (2)
A homogeneous mixture of Cu(NO3)2·2.5H2O (30 mmol), acetic acid (20 mL), and acetic anhydride (30 mL) was prepared at room temperature. To this solution were added carbazole (25 mmol) in small portions over 10 min. Temperature was maintained at 15-20 °C during addition of carbazole. The temperature was allowed to rise to room temperature over a period of 30 min and then to 90 °C. Reaction was continued with stirring for a period of 30 min at this temperature. The mixture was poured into 250 mL of distilled water with constant stirring. The precipitate was collected by filtration, and washed five times each with about 100 mL of distilled water. The filtrate was dried in vacuumat. Chromatography on silica gel, eluting with petroleum/EtOAC (3:1) gave 2 (5.23 g, 81%) as a yellow solid. mp >300 °C (lit.27 mp >300 °C). 1H NMR (400 MHz, DMSO-d6) δ 12.69 (s, 1H), 9.50 (d, J = 2.1 Hz, 2H), 8.40 (dd, J = 9.0, 2.1 Hz, 2H), 7.77 (d, J = 9.0 Hz, 2H). ESI-MS m/z: 258[M+H]+. | 81% |