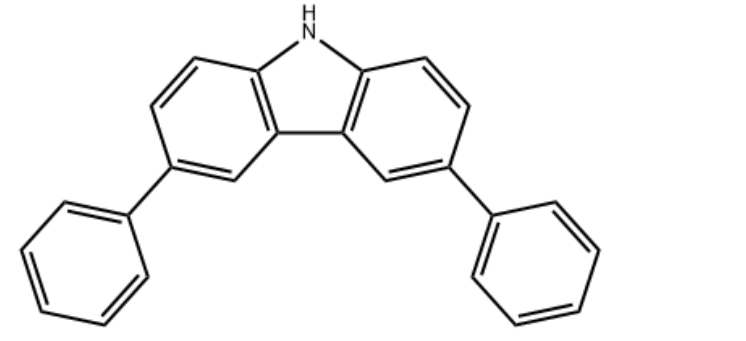
3,6-Diphenyl-9H-carbazole CAS#: 56525-79-2; ChemWhat Code: 1411497
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN114656396 | Organic compound and application thereof, and organic electroluminescent device containing organic compound | 2022 |
| KR2021/75622 | Novel compound and organic light emitting device comprising the same | 2021 |
| KR2021/75621 | Novel compound and organic light emitting device comprising the same | 2021 |
| KR2021/75620 | Novel compound and organic light emitting device comprising the same | 2021 |
| CN112876406 | Deuterated carbazole compound, preparation method thereof, photoelectric material and medicine | 2021 |
Physical Data
| Appearance | White powder |
| Melting Point, °C | Solvent (Melting Point) |
| 177.1 | |
| 186 | hexane, ethyl acetate |
| 186 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
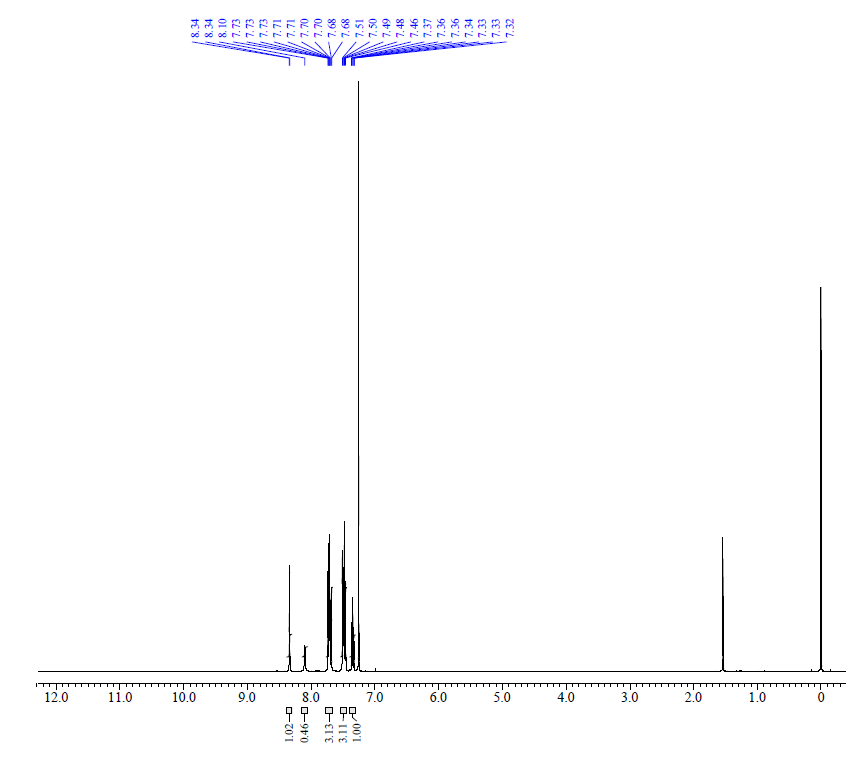
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 600 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 151 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | N,N-dimethyl-formamide |
| Spectrum | toluene |
| Spectrum | tetrahydrofuran |
Route of Synthesis (ROS)
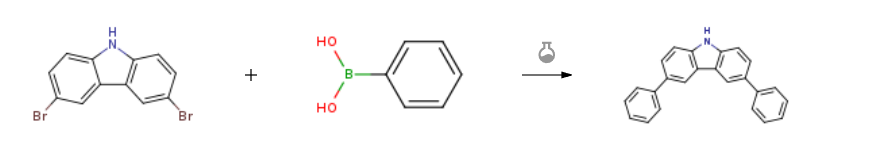
Route of Synthesis (ROS) of 3,6-Diphenyl-9H-carbazole CAS 56525-79-2
| Conditions | Yield |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane; water at 90℃; | 86% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane at 90℃; for 24h; Inert atmosphere; | 86% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 80℃; for 20h; Inert atmosphere; Experimental Procedure Add 3,6-dibromocarbazole (9.75g, 30.00mmol, 1.0 equivalent) to a dry three-necked flask with a magnetic stirring rotor and condenser.Phenylboronic acid (8.78g, 72.00mmol, 2.4 equivalents),Tetrakis(triphenylphosphine)palladium (347mg, 0.3mmol, 10mol%),Potassium carbonate (20.73g, 150.00mmol, 3 equivalents),Then pump nitrogen three times,Add toluene/ethanol/water (30mL/30mL/10mL) under nitrogen protection.The mixture was stirred and reacted in an oil bath at 80°C for 20 hours.TLC monitors until the reaction of the raw materials is complete, and cool to room temperature.A small amount of water was added and extracted twice with dichloromethane.The organic phases were combined, dried over anhydrous sodium sulfate, and filtered.The solvent was distilled off under reduced pressure.The obtained crude product is separated and purified by silica gel chromatography column, eluent: petroleum ether/ethyl acetate=20:1-5:1,The intermediate 3,6-diphenylcarbazole was obtained, 7.95 g of light brown solid, and the yield was 83%. | 83% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (33.33%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (33.33%): May cause an allergic skin reaction [Warning Sensitization, Skin] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (33.33%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P272, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P333+P313, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 319.406 |
| logP | 7.411 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 15.79 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Effect |
| 8.05 | pIC50(Virus replication) | = | 8.05 | antiviral agent | ||
| 8.05 | pIC50 | = | 8.05 | Topoisomerase dna ii 180kda (Beta) [Human immunodeficiency virus 1]:Wild | ||
| 5.65 | EC90(Amount Of P24 Protein) | > | 20 | µM | ||
| 5.48 | IC50(protease activity) | 3.3 | µM | |||
| 4.63 | IC50 | 23.45 | μg | antifungal agent | ||
| 4.59 | IC50 | 2.45 | μg/ml | antifungal agent | ||
| 4.45 | IC50 | 3.33 | μg/ml | antifungal agent | ||
| 4 | stimulation rate | Active | High voltage-activated calcium channel [rat]:Wild | |||
| 3.44 | IC50 | 34.55 | μg/ml | antifungal agent |
| Quantitative Results | ||
| 1 of 10 | Effect | inhibitory activity |
| Target | D-amino-acid oxidase [human]:Wild | |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory concentration of compound against human recombinant D-amino acid oxidase (DAO) expressed in Escherichia coli BL21(DE3)pLysS cells upon incubation in 40 mM sodium pyrophosphate, pH 8.3 for 10 mins at 37 degree C using 10 mM D-Alanine as substrate | |
| 2 of 10 | Assay Description | Clog P value of the compound was calculated using Hansch’s LogP |
| Measurement | Clog P value of the compound | |
| 3 of 10 | Assay Description | Dissociation constant of the compound was measured by using Hammett’s equation |
| Measurement | Dissociation constant | |
| 4 of 10 | Assay Description | Hydrogen bond acidity of the compound was determined |
| Measurement | Hydrogen bond acidity | |
| 5 of 10 | Target | Fatty-acid amide hydrolase 1:Wild |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory actvity of the compound against Fatty acid amide hydrolase in 0.1 M sodium phosphate, pH 8.0 | |
| 6 of 10 | Assay Description | Acid dissociation constant of compound was determined |
| Measurement | Acid dissociation constant | |
| 7 of 10 | Biological material | CEM-T4 cell line |
| Assay Description | Selectivty index of compound was measured as Cytotoxic concentration against mock infected CEM-T4 cells to that of effective concentration required to acieve HIV induced cytopathogenicity | |
| Results | SI50 not calculated | |
| Measurement | SI50 | |
| 8 of 10 | Effect | Genotoxic |
| Biological material | HL-60 cell line | |
| 9 of 10 | Effect | antibiotic agent |
| Biological material | Staphylococcus aureus | |
| Assay Description | Effect : antistaphylococcal | |
| 10 of 10 | Results | effect on phosphatidylcholine secretion in primary cultures of rat type II pneumocytes |
| Use Pattern |
| 3,6-Diphenyl-9H-carbazole CAS#: 56525-79-2 is an important organic compound with versatile applications. Firstly, it finds extensive use in the field of optoelectronics. 3,6-Diphenyl-9H-carbazole is a fluorescent material and can be employed in the fabrication of organic light-emitting diodes (OLEDs). |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to [email protected] |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |