4-Hydroxybenzyl alcohol CAS#: 623-05-2; ChemWhat Code: 66883
Identification
| Product Name | 4-Hydroxybenzyl alcohol |
| IUPAC Name | 4-(hydroxymethyl)phenol |
| Molecular Structure | 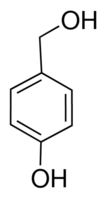 |
| CAS Registry Number | 623-05-2 |
| EINECS Number | 210-768-0 |
| MDL Number | MFCD00004658 |
| Beilstein Registry Number | 1858967 |
| Synonyms | (4-hydroxyphenyl)methanol, 4-Hydroxybenzyl alcohol; CAS No.: 623-05-2 |
| Molecular Formula | C7H8O2 |
| Molecular Weight | 124.137 |
| InChI | InChI=1S/C7H8O2/c8-5-6-1-3-7(9)4-2-6/h1-4,8-9H,5H2 |
| InChI Key | BVJSUAQZOZWCKN-UHFFFAOYSA-N |
| Canonical SMILES | c1cc(ccc1CO)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109384644 | A method of synthesis of primary alcohol (by machine translation) | 2019 |
| CN108456178 | With the neuroprotective activity of Rhizoma Chuanxiong hydrochlorothizide substituted P-hydroxy-methanol analogs derivatives (LQC – F) and its application (by machine translation) | 2018 |
| CN107253894 | Halogenated aromatic compound hydroxylated method (by machine translation) | 2017 |
| US2013/79532 | PROCESS FOR THE PREPARATION OF 2-HYDROXY-4-PHENYL-3,4-DIHYDRO-2H-CHROMEN-6-YL-METHANOL AND (R)-FESO-DEACYL | 2013 |
| US2014/135524 | PERFLUOROPOLYVINYL MODIFIED ARYL INTERMEDIATES/MONOMERS | 2014 |
Physical Data
| Appearance | White to off-white to yellow to cream colored crystalline powder |
| Solubility | Soluble in water (6.7 mg/ml at 20°C), dioxane (100 mg/ml), 1N NaOH (50 mg/ml), DMSO, and methanol. |
| Flash Point | 251-253°C |
| Refractive index | 1.5035 (estimate) |
| Sensitivity | Light Sensitive/Air Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 120 – 122 | |
| 118 – 119 | |
| 110 – 112 | |
| 119 – 123 | |
| 43 | |
| 125 – 126 | methanol |
| 125 | benzene, ethanol |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.14 | 4 | 25 |
| 1.2 | 4 | -190 |
| 1.24 |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| Adsorption | titanium(IV) oxide |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | chloroform-d1 | 400 | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 | |
| Chemical shifts | 1H | water-d2 | 400 | |
| Chemical shifts, Spectrum | 13C | d(4)-methanol | 100 | |
| Spectrum | 1H | dimethylsulfoxide-d6 | ||
| Spin-spin coupling constants | D2O, CD3CN | |||
| NMR |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | film |
| Spectrum | potassium bromide | |
| Bands | KBr | 3360 – 1515 cm**(-1) |
| IR | ||
| Spectrum | nujol | 1053 – 690 cm**(-1) |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum |
| electrospray ionisation (ESI), spectrum |
| liquid chromatography mass spectrometry (LCMS), tandem mass spectrometry, electrospray ionisation (ESI), IT (ion trap), spectrum |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
| spectrum, electron impact (EI) |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| gas chromatography mass spectrometry (GCMS), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | water | |||
| Absorption maxima | ethanol | 225, 277, 283 | 8511, 1549, 1318 | |
| H2O | in the presence of inorganic compounds | 273 | ||
| Absorption maxima | aq. NaOH | 275 | ||
| UV/VIS |
| Description (Fluorescence Spectroscopy) |
| Spectrum, Maxima |
| Fluorescence |
Route of Synthesis (ROS)
| Conditions | Yield |
| With potassium carbonate In acetone for 4h; Heating; | 96% |
| With sodium hydride In N,N-dimethyl-formamide at 0 – 20℃; for 6h; Inert atmosphere; | 93% |
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 6h; | 90% |
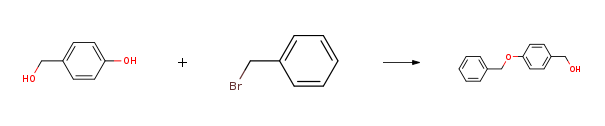
| Stage #1: (4-hydroxyphenyl)methanol With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 20℃; for 20h; | 87% |
| With sodium hydride 1.) DMF, RT, 1 h, 2.) DMF, RT, 6 h; Yield given. Multistep reaction; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (29.09%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (27.88%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 290729 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 124.139 |
| logP | 0.805 |
| HBA | 1 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.46 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 5.52 | current density decrease(M-type K+ current (IK(M)) taken from -50 to -10 mV) | Active | |||
| 5.25 | Ki (inhibition constant) | 5.6 | µM | antibiotic agent | |
| 5.21 | Ki (inhibition constant) | 6.1 | µM | ||
| 5.11 | IC50 | 7.7 | µM | antiproliferative agent | |
| 5.07 | Ki (inhibition constant) | 8.6 | μM | antibiotic agent | |
| 4.93 | Ki (inhibition constant) | 10.5 | μM | antibiotic agent | |
| 4.92 | Kd (dissociation constant)(at -50 to -10 mV) | 11.9 | μM | antifungal agent | |
| 4.62 | inhibition percentage(cell death) | 80.8 | % | neuroprotective agent | |
| 4 | protein expression level decrease | Active | μg/ml | antifungal agent |
| Quantitative Results | ||
| 1 of 10 | Biological material | human skin |
| Assay Description | Permeability coefficient of the compound in human skin was determined | |
| Measurement | Permeability coefficient | |
| 2 of 10 | Biological material | rat |
| Assay Description | Dose of the compound required to inhibit arachidonic acid-induced increase in the ear thickness in rats measured 40 min after induction of inflammation | |
| Results | Dose not calculated | |
| Measurement | Dose | |
| 3 of 10 | Assay Description | Partition coefficient of the compound in Octanol-water medium |
| 4 of 10 | Biological material | Anura skin |
| Assay Description | Relative biological activity of compound determined in frog skin as compared to that of 2-hydroxybenzyl alcohol | |
| Results | log RBR not calculated | |
| Measurement | log RBR | |
| 5 of 10 | Target | 4-cresol dehydrogenase [hydroxylating] flavoprotein subunit:Wild |
| Assay Description | Specific activity of compound towards p-Cresol methylhydroxylase (PCMH) from denitrifying bacterial isolate (PC-07) using 100 umol 2,6-dichlorophenol-indophenol (DCPIP) upon incubation in 50 mM Tris-HCl buffer, pH 7.6 by colorimetric DCPIP-PMS assay; One unit is equivalent to 1.0 umol of DCPIP reduced per min per mg of protein | |
| Results | Specific activity not calculated | |
| Measurement | Specific activity | |
| 6 of 10 | Assay Description | Apparent partition coefficient (LogP) value of the compound was determined |
| Measurement | Partition coefficient | |
| 7 of 10 | Effect | Cytotoxic |
| Assay Description | Target : Vitis vinifera cv. Chasselas cells Bioassay : minimal NOVER-MSMO medium; incubated for 24 h at 24 deg C; cell death and membrane integrity assessed using vital dye neutral red; accumulation of dye in vacuoles and plasmolysis observed by light microscopy | |
| 8 of 10 | Target | 4-aminobutyrate aminotransferase, mitochondrial:Wild |
| Assay Description | Effect : enzyme; inhib. of Bioassay : valproic acid (known anticonvulsant) used as reference comp. γ-aminobutyric acid; α-ketoglutaric acid; assay buffer, pH 8.0; 37 deg C; incubated for 30 min; NADP added and amount of NADPH generated for 20 min measured at 340 nm as activity of enzyme | |
| Results | title comp. inhibited enzyme activity by 30.87 percent (vs. 65.38 percent inhibition for valproic acid) | |
| 9 of 10 | Effect | Phytotoxic |
| Biological material | cucumber | |
| Assay Description | Bioassay : root elongation half inhibition concentration (RC50) was investigated after 48 h of incubation in the dark at 25 +/- 1 deg C, the root elongation of each seed was measured to 1 mm | |
| Results | RC50 309.0 mg/l | |
| 10 of 10 | Effect | Germination Effect |
| Biological material | cucumber | |
| Assay Description | Bioassay : GC50: negative logarithm of germination rate 50 percent inhibition concentration in mol/l seeds purchased commercially; 100×15 mm disposable petri dishes; Whatman No. 1 filter paper; incubation time 48 h; 25 deg C; in the dark; pH 6.15; OECD, 1984 | |
| Results | GC50 3.20 dimensionless |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 5 | MIC | 10.1 | µM | ||
| 3 | percentage increase(of cellular protection) | Active | cytoprotective agent | ||
| 2.7 | IC50 | 2 | mM | antiproliferative agent | |
| 2.66 | IC50 | 2.2 | mM | antiproliferative agent | |
| inhibition percentage(of oxidized DCFH-DA to fluorescent DCF) | Moderate | Cytotoxic |
| 1 of 2 | Effect | Toxic |
| Biological material | Tetrahymena pyriformis | |
| Assay Description | Effect : growth Bioassay : spectrophotometric, 540 nm; sterile medium; 27 deg C; pH 7.35; starting conc. of cells: ca. 2500 cells/ml; conc. of title comp.: saturated solution | |
| 2 of 2 | Effect | Cytotoxic |
| Assay Description | Target : murine macrophage RAW264.7 cells Bioassay : controls: cells incub. in absence of title comp.; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cells incub. with title comp. then treated with MTT for 2 h; cell viability determ. by MTT reduction assay |
| Use Pattern |
| 4-Hydroxybenzyl alcohol CAS#: 623-05-2 Agricultural use |
| 4-Hydroxybenzyl alcohol CAS#: 623-05-2 controlling nematodes |
| 4-Hydroxybenzyl alcohol CAS#: 623-05-2 in combination with 2-Methyl-2-(methylthio)propanal O- (N-methylcarbamoyl)oxime, 2,2-Dimethyl-2,3-dihydro-l-benzofuran-7-yl methylcarbamate, Methyl 2- (dimethylamino)-N-[(methylcarbamoyl)oxy]-2-oxoethanimidothioate, 2-Methyl-2- (methylsulfonyl)propionaldehyde 0-(methylcarbamoyl)oxime, Ο,Ο-Diethyl 0-[4- (methylsulfinyl)phenyl] phosphorothioate, l-(ethoxy-propylsulfanylphosphoryl)sulfanylpropane, (RS)-N-[Ethoxy-(3-methyl-4-methylsulfanylphenoxy)phosphoryl]propan-2-amine, Streptomyces lydicus WYEC |
| 4-Hydroxybenzyl alcohol CAS#: 623-05-2 in combination with Sinapsis alba plant extract, Sinapsis alba seed meal, or a combination thereof |
| 4-Hydroxybenzyl alcohol CAS#: 623-05-2 in combination with potato hatching factor, a potato root diffusate, a tomato root diffusate, a soybean root diffusate, a sugar beet root diffusate, or any combination thereof |
| 4-Hydroxybenzyl alcohol CAS#: 623-05-2 therapeutic component of pharmaceutical compoosition |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |