4-Hydroxyindole CAS#: 2380-94-1; ChemWhat Code: 211762
Identification
| Product Name | 4-Hydroxyindole |
| IUPAC Name | 1H-indol-4-ol |
| Molecular Structure | 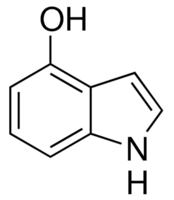 |
| CAS Registry Number | 2380-94-1 |
| EINECS Number | 219-177-2 |
| MDL Number | MFCD00005667 |
| Beilstein Registry Number | 114905 |
| Synonyms | 1H-indol-4-ol, 4-hydroxyindole |
| Molecular Formula | C5H6N2 |
| Molecular Weight | 94.116 |
| InChI | InChI=1S/C8H7NO/c10-8-3-1-2-7-6(8)4-5-9-7/h1-5,9-10H |
| InChI Key | NLMQHXUGJIAKTH-UHFFFAOYSA-N |
| Canonical SMILES | c1cc2c(cc[nH]2)c(c1)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109678784 | indole compound and its preparation method (by machine translation) | 2019 |
| WO2019/213295 | SMALL MOLECULE MODULATORS OF MHC-I | 2019 |
| WO2006/18850 | COMPOSITIONS AND METHODS USING SAME FOR TREATING AMYLOID ASSOCIATED DISEASES | 2006 |
| US2003/229069 | 1-SULFONYL-4-AMINOALKOXY INDOLE DERIVATIVES AND USES THEREOF | 2003 |
Physical Data
| Appearance | Light brown to off-white crystalline powder |
| Solubility | slightly soluble |
| Refractive index | 1.5260 (estimate) |
| Sensitivity | Air Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 94 – 97 | cyclohexane |
| 102 – 104 | |
| 93 – 94.5 | CHCl3, hexane |
| 97 – 99 | H2O |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 150 | 4 |
| 100 | 0.01 |
| Description (Association (MCS)) | Partner (Association (MCS)) |
| Association with compound | copper(II) ion |
| Further physical properties of the complex | triethylamine |
| Further physical properties of the complex | H2O |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | ||
| 1H | chloroform-d1 | 500 | 1H-NMR (500MHz, CDCl3, δppm): 8.19 (br.s, 1H), 7.13 (m, 1H), 7.06 (dd,J= 8.0, 7.6 Hz, 1H), 7.01 (d,J= 8.0 Hz, 1H), 6.62 (m, 1H), 6.54 (d,J= 7.6 Hz, 1H), 5.22 (br.s, 1H). | |
| 13C | chloroform-d1 | 126 | 13C-NMR (126MHz, CDCl3, δppm): 149.08, 137.86, 123.18, 123.03, 117.64, 104.37, 104.29, 98.91. | |
| Chemical shifts | 1H | CDCl3 | 400 | |
| Chemical shifts | 13C | CDCl3 | ||
| Spin-spin coupling constants | CDCl3 | |||
| Chemical shifts | 13C | acetone-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | CH2Cl2 | 3440 – 1364 cm**(-1) |
| Bands | CDCl3 | 3620 – 1460 cm**(-1) |
| Spectrum | nujol | 5000 – 714 cm**(-1) |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| HRMS (High resolution mass spectrometry), LCMS (Liquid chromatography mass spectrometry), TOFMS (Time of flight mass spectrum), EI (Electron impact), Spectrum | |
| electron impact (EI) | IKE(S) (ion kinetic energy (spectrum)) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | cyclohexane | |||
| Absorption maxima | methanol, aq. H2SO4 | 282 | 5012 | |
| Absorption maxima | methanol, aq. KOH | 299 | 8318 | |
| Absorption maxima | methanol | 218, 264, 280, 291 | ||
| Absorption maxima | methanol, aq. KOH | Remark: 25.0 deg C | 291.4, 267.6, 293.2 | 6457, 6918, 8318 |
| UV/VIS | ||||
| Spectrum | ethanol | 200 – 320 nm |
Route of Synthesis (ROS)
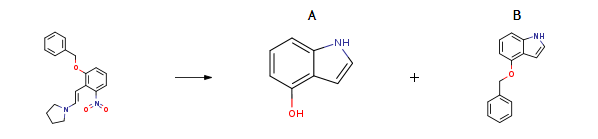
| Conditions | Yield |
| With hydrogen; 5% rhodium-on-charcoal; nickel(II) nitrate In tetrahydrofuran; water at 20℃; for 32h; | A 6% B 88% |
| With hydrogen; 5% rhodium-on-charcoal; iron(II) acetate In tetrahydrofuran at 20℃; for 31h; | A 5% B 83% |
| With hydrogen; 5% rhodium-on-charcoal; tris(acetylacetonato)cobalt In tetrahydrofuran at 20℃; for 38h; | A 3% B 80% |
| With hydrogen; 5% rhodium-on-charcoal In tetrahydrofuran at 20℃; for 167h; | A 38% B 43% |
| With hydrogen; Rh on carbon; nickel(II) nitrate In tetrahydrofuran at 20℃; | A 6 % Chromat. B 88 % Chromat. |
| With hydrogen; Rh on carbon In tetrahydrofuran at 20℃; | A 38 % Chromat. B 43 % Chromat. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (97.96%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (93.88%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (95.92%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 296200 |
| Storage | Store below 10°C for long time, sealed, keep in a dry place and away from light. |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 133.15 |
| logP | 1.769 |
| HBA | 1 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 36.02 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Tissue/Organ |
| 4.96 | Ki (inhibition constant) | 10.87 | µM | Odorant-binding protein 1 [Maruca vitrata]:Wild | ||
| 4.84 | IC50 | 14.62 | µM | Odorant-binding protein 1 [Maruca vitrata]:Wild | ||
| 4.63 | inhibition percentage | 30 | % | 5-hydroxytryptamine receptor [Leporidae]:Wild | gastrointestinal tract | |
| 3.7 | IC50 | = | 200 | µM | ||
| 3.7 | IC50(Beta-amyloid fibril formation) | = | 200 | µM | ||
| 3.7 | Ki (inhibition constant) | <= | 500000 | nM | indoleamine 2,3-dioxygenase [human]:Wild | |
| LD50 | 620 | μg |
| Quantitative Results | ||
| 1 of 8 | Target | indoleamine 2,3-dioxygenase [human]:Wild |
| Assay Description | Effect : indoleamine-2,3-dioxygenase (IDO); inhibition of Bioassay : Example 13 – In Vitro Recombinant IDO Enzyme Activity Assays.The effect of extracts, purified compounds and synthesized compounds on IDO activity was determined with the use of recombinant human IDO expressed in E. coli(Vottero et al. 2006. Biotechnology J. 1:282-288) and purified (Sugimoto et al. 2006. | |
| 2 of 8 | Biological material | human |
| Assay Description | Effect : indoleamine-2,3-dioxygenase (IDO); inhibition of Bioassay : indoleamine-2,3-dioxygenase (IDO) Example 13 – In Vitro Recombinant IDO Enzyme Activity Assays.The effect of extracts, purified compounds and synthesized compounds on IDO activity was determined with the use of recombinant human IDO expressed in E. coli(Vottero et al. 2006. Biotechnology J. 1:282-288) and purified (Sugimoto et al. 2006. | |
| 3 of 8 | Target | enzyme:Wild |
| Assay Description | Effect : enzyme; inhib. of Bioassay : formation of <14C>guanosine 5′-monophosphate determined in vitro; inhibition of T. gondii guanine phosphoribosyltransferase activity evaluated; 4 μmol/l <8-14C>guanine (spec. act.: 55 Ci/mol); 4 mmol/l 5′-phosphoribosyl-1-pyrophosphate; assay buffer, pH 7.4; 37 deg C | |
| Results | at 0.9 mmol/l title comp. produced less than 10 percent inhibition of enzyme activity | |
| 4 of 8 | Target | enzyme:Wild |
| Assay Description | Effect : enzyme; inhib. of Bioassay : formation of <14C>xanthosine 5′-monophosphate determined in vitro; inhibition of T. gondii xanthine phosphoribosyltransferase activity evaluated; 10 μmol/l <8-14C>xanthine (spec. act.: 55 Ci/mol); 4 mmol/l 5′-phosphoribosyl-1-pyrophosphate; assay buffer, pH 7.4; 37 deg C | |
| Results | at 0.9 mmol/l title comp. produced less than 10 percent inhibition of enzyme activity | |
| 5 of 8 | Effect | cytoprotective agent |
| Biological material | PC12 cell line (pheochromocytoma) | |
| Assay Description | Bioassay : MATERIALS AND EXPERIMENTAL METHODS Cell culture: PC 12 pheochromocytoma cell line, a cultured benign tumor of the sympathetic nervous system, was routinely grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 8 % (v/v) fetal calf serum, 8 % (v/v) horse serum, 100 U/ml penicillin, | |
| Results | title compound induced dose-dependent increase in survival of cells treated with β-amyloid polypeptide (Aβ 1-40) on day 0 and 7 of incubation (figure is given) | |
| 6 of 8 | Results | antifouling activity |
| 7 of 8 | Effect | Behavioural Symptoms |
| Assay Description | Target : ICR albino mouse Bioassay : in vivo; 6 mice (25-30 g, food and water ad libitum); drug administered intracranially; blood parameters: Hb, RBC, WBC, cholesterol, sugar, urea, BUN, alkaline phosphatase, ALT, and AST determined after 1 day, 3 days, 1 week, and 2 weeks | |
| Results | Hb,RBC, and WBC not affected by drug; the value of cholesterol, sugar, and blood urea decreased significantly after 1 day; cholesterol reached the normal value after 2 weeks, other parameters remained lower; ALT, AST and phosphatase values increased | |
| 8 of 8 | Results | increase of estradiol C-2 hydroxylase activity in MCF-7 cells, only small effect on estradiol C-16α hydroxylase activity |
| Use Pattern |
| 4-Hydroxyindole CAS#: 2380-94-1 BRCA1-deficient breast cancer |
| 4-Hydroxyindole CAS#: 2380-94-1 BRCA2-deficient breast cancer |
| 4-Hydroxyindole CAS#: 2380-94-1 PALB2-deficient cancer |
| 4-Hydroxyindole CAS#: 2380-94-1 as Pharmaceuticals |
| inhibits RAD52 annealling of RPA-coated ssDNA |
| coupler for dyeing keratin fibers |
| coupler in composition for oxidation dyeing of keratin fibres |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

