4-(HYDROXYMETHYL)PHENYLACETIC ACID CAS#: 73401-74-8; ChemWhat Code: 1119205
Identification
| Product Name | 4-(HYDROXYMETHYL)PHENYLACETIC ACID |
| IUPAC Name | 2-[4-(hydroxymethyl)phenyl]acetic acid |
| Molecular Structure | 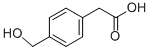 |
| CAS Registry Number | 73401-74-8 |
| NACRES | NA.22 |
| MDL Number | MFCD00065692 |
| Synonyms | 4-hydroxymethylphenylacetic acid, 4-Hydroxymethylphenylacetic acid, 2-(4-(hydroxymethyl) phenyl) acetic acid, 2-(4-(hydroxymethyl)phenyl)acetic acid, 2-[4-(hydroxymethyl)phenyl]acetic acid, [4-(hydroxymethyl)phenyl]acetic acid, 4-(hydroxymethyl)phenylacetic acid |
| Molecular Formula | C9H10O3 |
| Molecular Weight | 166.177 |
| InChI | InChI=1S/C9H10O3/c10-6-8-3-1-7(2-4-8)5-9(11)12/h1-4,10H,5-6H2,(H,11,12) |
| InChI Key | FWZBPBKAANKOJQ-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC(=CC=C1CC(=O)O)CO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/121250 | ALKYLAMIDOTHIAZOLES, COSMETIC OR DERMATOLOGICAL PREPARATIONS CONTAINING SAID ALKYLAMIDOTHIAZOLES, AND USE THEREOF TO COMBAT OR PREVENT UNDESIRED PIGMENTATION OF THE SKIN | 2014 |
| US2014/161736 | COMPOUNDS HAVING MUSCARINIC RECEPTOR ANTAGONIST AND BETA2 ADRENERGIC RECEPTOR AGONIST ACTIVITY | 2014 |
| WO2013/52110 | NOVEL MOLECULES THAT SELECTIVELY INHIBIT HISTONE DEACETYLASE 6 RELATIVE TO HISTONE DEACETYLASE 1 | 2013 |
| US2002/173051 | Solid phase synthesis supports and methods | 2002 |
Physical Data
| Appearance | White to off-white powder |
| Melting Point, °C |
| 128.5 – 129 |
| 131-134 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
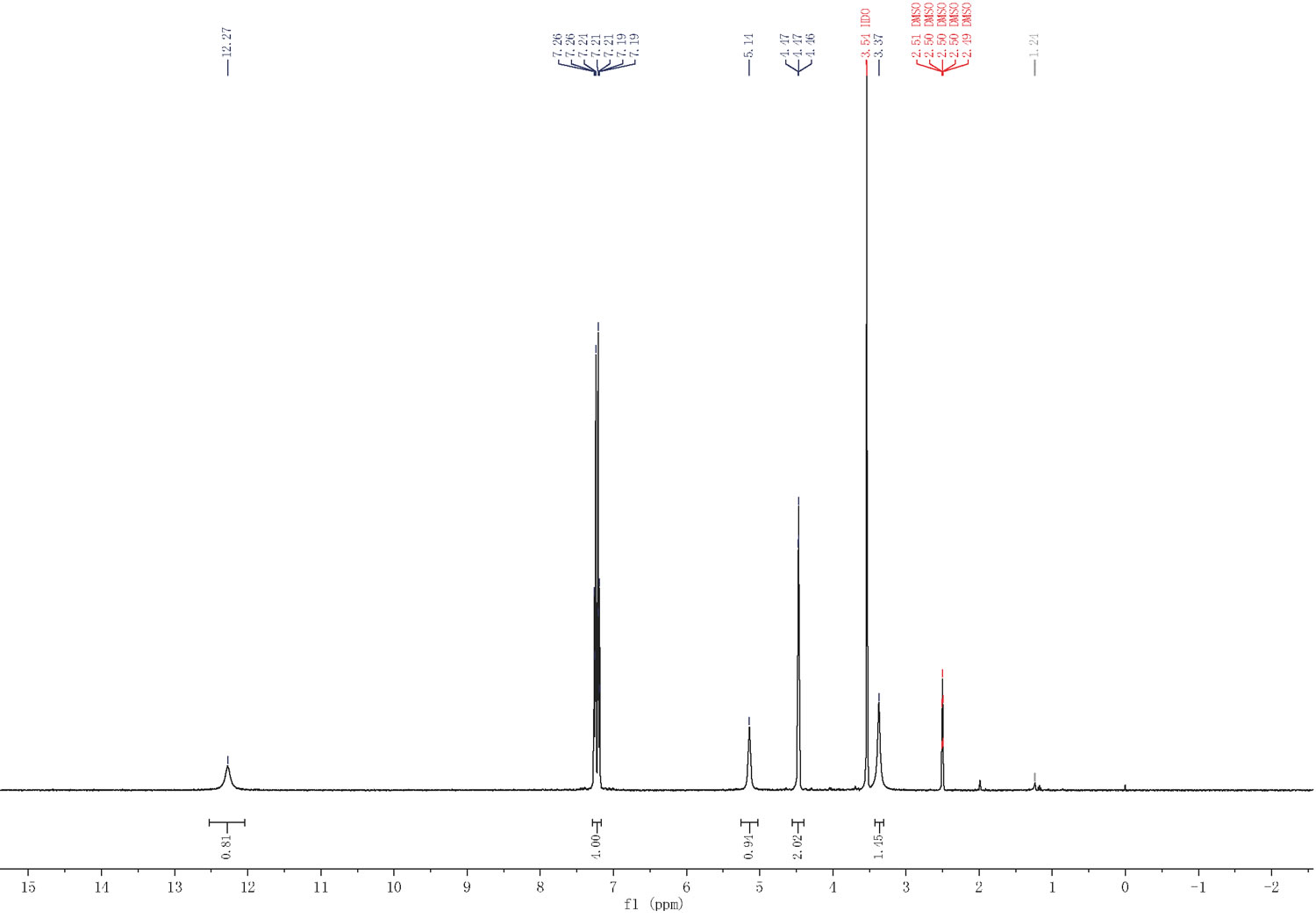
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | 1H NMR (300 MHz, DMSO-d6) δ 12.27 (s, 1H), 7.26-7.12 (m, 4H), 5.14 (s, 1H), 4.47 (s, 2H), 3.53 (s, 2H). |
| Original Text (IR Spectroscopy) |
| νmax cm-1: 3304, 1703, 1520, 1421, 1408, 1360, 1288, 1234, 1200, 1188, 1009 |
Route of Synthesis (ROS)

| Conditions | Yield |
| With sodium hydroxide In water at 18 – 25℃; Large scale; Experimental Procedure 1 2-(4-(Hydroxymethyl)phenyl)acetic acid (C) 2-(4-(Hydroxymethyl)phenyl)acetic acid (C) To a solution of NaOH (1.61 kg, 40.2 mol, 4 eq) in water (90 L) was added B (2.3 kg, 10.0 mol, 1 eq) and the resulting mixture was stirred at RT overnight. TLC analysis indicated consumption of B. The reaction mixture was then carefully acidified with concentrated H2SO4 (1.0 L) to pH˜2. Then, solid NaCl (25 kg) was added to the mixture followed by extraction with EtOAc (33 L3). The combined organic phase was washed with brine, dried over Na2SO4, and concentrated until a significant amount of solid precipitated. The resulting suspension was kept at ˜4-6° C. overnight to allow for further crystallization. The solid product was then collected by filtration. The filter cake was washed with petroleum ether (2 L2) to yield the title compound (1.2 kg, yield: 71.9%). HPLC purity: 97.8% (220 nm); 1H NMR (300 MHz, DMSO-d6) δ 12.27 (s, 1H), 7.26-7.12 (m, 4H), 5.14 (s, 1H), 4.47 (s, 2H), 3.53 (s, 2H). | 71.9% |
| With sodium hydroxide In water at 20℃; Large scale; Experimental Procedure 1 2-(4-(Hydroxymethyl)phenyl)acetic acid (C): To a solution of NaOH (1.61 kg, 40.2 mol, 4 eq) in water (90 L) was added B (2.3 kg, 10.0 mol, 1 eq) and the resulting mixture was stirred at RT overnight. TLC analysis indicated consumption of B. The reaction mixture was then carefully acidified with concentrated H2SO4 (1.0 L) to pH ~2. Then, solid NaCl (25 kg) was added to the mixture followed by extraction with EtOAc (33 L x 3). The combined organic phase was washed with brine, dried over Na2S04, and concentrated until a significant amount of solid precipitated. The resulting suspension was kept at ~4-6°C overnight to allow for further crystallization. The solid product was then collected by filtration. The filter cake was washed with petroleum ether (2 L x 2) to yield the title compound (1.2 kg, yield: 71.9%). HPLC purity: 97.8% (220 nm); 1H NMR (300 MHz, OMSO-d6) δ 12.27 (s, 1H), 7.26-7.12 (m, 4H), 5.14 (s, 1H), 4.47 (s, 2H), 3.53 (s, 2H). | 71.9% |
| With sodium hydroxide; sodium chloride; sodium acetate In water Experimental Procedure 44.i 44(i) 44(i) 4-Hydroxymethylphenylacetic acid A solution of 9.00 g (39.3 mmol) of p-bromomethylphenylacetic acid, 3.14 g (78.6 mmol) of sodium hydroxide and 3.54 g (43.2 mmol) of sodium acetate in 40 ml of water was stirred for 4 hours at 100° C. At the end of this time, it was cooled to room temperature and then acidified with 2N aqueous hydrochloric acid. It was then extracted with ethyl acetate. The extract was washed with a saturated aqueous solution of sodium chloride. The organic phase was dried over anhydrous sodium sulfate, and the solvent was removed by distillation under reduced pressure. The resulting residue was recrystallized from a mixture of ethyl acetate and methylene chloride to give 3.60 g (yield 55%) of the title compound, melting at 136°-137° C. Infrared Absorption Spectrum (KBr) νmax cm-1: 3304, 1703, 1520, 1421, 1408, 1360, 1288, 1234, 1200, 1188, 1009. | 55% |
| With water for 2h; Reflux; Experimental Procedure 1 Synthetic compound 3 Compound 2 (50 g) was added to 200 ml of water,Stir and heat to reflux for 2 hours,Anti-strain clarification,Then cool to room temperatureThe ice bath cooled to about 2 degrees,A white solid precipitated and was filtered. The cake was washed twice with water to give a white solid.The white solid was dried at 50[deg.] C. for 10 hours to yield about 33 g of compound 3. | 33g |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 291819 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 166.177 |
| logP | 0.638 |
| HBA | 3 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 57.53 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 4-(HYDROXYMETHYL)PHENYLACETIC ACID CAS#: 73401-74-8 is usually as a linker in peptide synthesis. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |