4-Methylbenzenesulfonic anhydride CAS#: 4124-41-8; ChemWhat Code: 26658
Identification
| Product Name | 4-Methylbenzenesulfonic anhydride |
| IUPAC Name | (4-methylphenyl)sulfonyl 4-methylbenzenesulfonate |
| Molecular Structure | 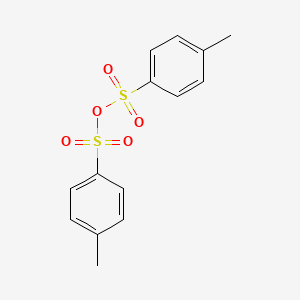 |
| CAS Registry Number | 4124-41-8 |
| EINECS Number | 223-926-9 |
| MDL Number | MFCD00008548 |
| Beilstein Registry Number | No data available |
| Synonyms | p-toluenesulfonylanhydridepara-toluenesulfonic anhydrideP-toluenesulfonic anhydrideTs2O4-methylbenzenesulfonic anhydridep-tolylsulfonyl 4-methylbenzenesulfonatetosic anhydride |
| Molecular Formula | C14H14O5S2 |
| Molecular Weight | 326.381 |
| InChI | InChI=1S/C14H14O5S2/c1-11-3-7-13(8-4-11)20(15,16)19-21(17,18)14-9-5-12(2)6-10-14/h3-10H,1-2H3 |
| InChI Key | PDVFSPNIEOYOQL-UHFFFAOYSA-N |
| Canonical SMILES | Cc1ccc(S(=O)(=O)OS(=O)(=O)c2ccc(C)cc2)cc1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2017/197912 | SYNTHESIZING PET TRACERS USING [F-18]SULFONYL FLUORIDE AS A SOURCE OF [F-18]FLUORIDE | 2017 |
| US2014/243372 | BENZOIC ACID DERIVATIVE MDM2 INHIBITOR FOR THE TREATMENT OF CANCER | 2014 |
Physical Data
| Appearance | Off-white solid |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C |
| 122 – 123 |
| 125 |
| 99 – 101 |
| 101 – 103 |
Spectra
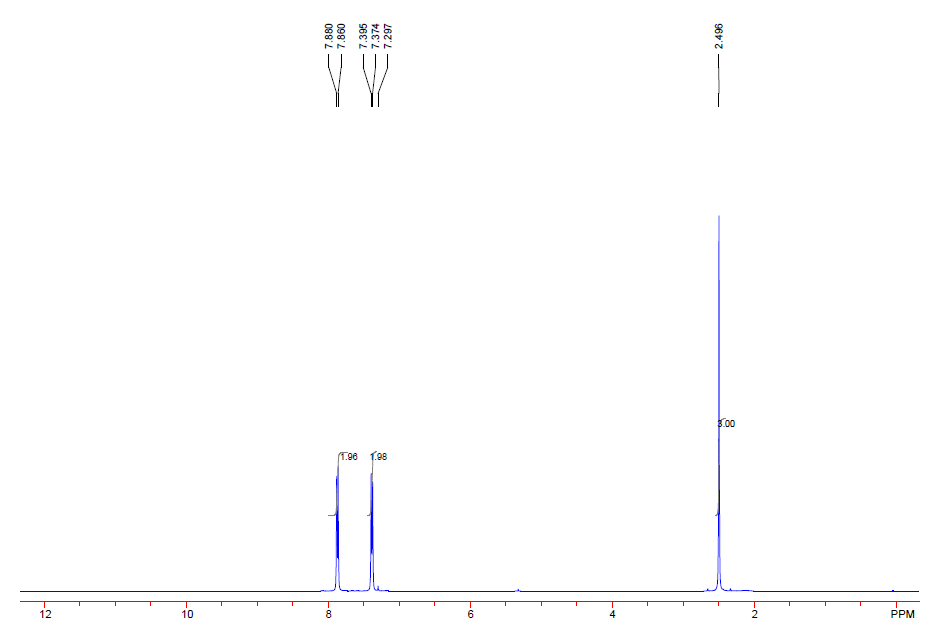
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 200 |
| Chemical shifts | 13C | chloroform-d1 | 50 |
| Description (IR Spectroscopy) |
| IR |
| Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With sodium hydroxide In lithium hydroxide monohydrate at 20℃; for 0.166667h; Inert atmosphere; | 80% |
| With sodium hydroxide In lithium hydroxide monohydrate at 20℃; for 0.166667h; | 42% |
| With sodium hydroxide In lithium hydroxide monohydrate regioselective reaction; | 26% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Store in a dry place at 16° for a long time. The product is easy to absorb moisture and needs to be stored with desiccant. |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 326.394 |
| logP | 2.505 |
| HBA | 5 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 94.27 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Organic synthesis – strong dehydration condensation agent Esterification reaction Activate carboxyl to accelerate ester formation Peptide bond formation Mildly catalyze amino acid condensation Sulfonylation protecting group Introduce -SO₂Tol group to protect amino/hydroxyl groups |
| Synthesis of pharmaceutical intermediates Antiviral drugs, synthesis of acyclovir side chain Reaction yield increased >15%(compared with methanesulfonyl chloride method) Antitumor drugs, construction of temozolomide pyrimidine ring Avoid the use of highly toxic phosgene Antibiotics, modification of β-lactam ring C3 position Selectivity >90%(such as cefixime precursor) |
| Polymer material modification Polyester catalyst Replacement of antimony oxide (reducing heavy metals) Material transmittance ↑8%, melting point stability improved Epoxy resin curing accelerator Activate curing reaction at low temperature (60℃) Curing time shortened 40% Ionic liquid synthesis Preparation of sulfonic acid ionic liquid Conductivity ≥15 mS/cm (fuel cell electrolyte) |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |