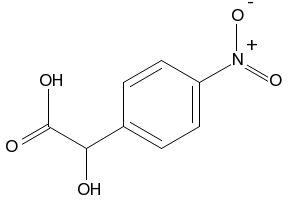
4-nitrophenylglycolic acid CAS#: 10098-39-2; ChemWhat Code: 128786
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US3985738 | 7-(D-α-Hydroxy-2-arylacetamido)-3-(tetrazolo-[4,5-b]pyridazin-6-ylthiomethyl)-3-cephem-4-carboxylic acids | 1976 |
| US4112228 | 7-(D-α-Hydroxy-2-arylacetamido)-3-(2-carboxyalkyl-2,3-dihydro-s-triazolo-[4,3-b]pyridazin-3-on-6-ylthiomethyl)-3-cephem-4-carboxylic acids and derivatives | 1978 |
Physical Data
| Appearance | Off-white solid |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 238 – 240 | |
| 160 – 161 | |
| 130 | diethyl ether, benzene |
| 126 – 127 | H2O |
| 126 – 127 | toluene |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | aq. acetic acid | 14.85 – 29.85 | bis(2,2′-bipyridyl)copper(II) permangamate |
| Stability constant of the complex with … | 29.9 – 59.9 | benzyltrimethylammonium tribromide | |
| Stability constant of the complex with … | dimethylsulfoxide | 14.9 – 44.9 | pyridinium fluorochromate |
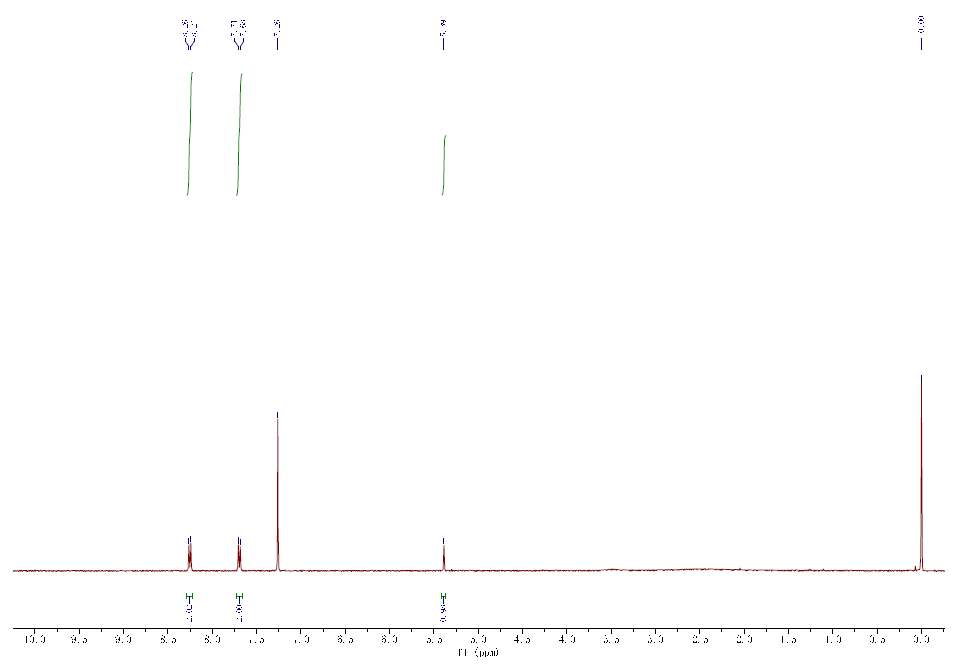
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 75 |
| 1H | tetradeuteriomethanol |
| Description (IR Spectroscopy) |
| Bands |
| Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| neutral aq. solution | 285 | 10100 | ||
| Absorption maxima | H2O | Ratio of solvents: 0.1N | 280 | 9300 |
| Spectrum | H2O | Remark: pH 7 |
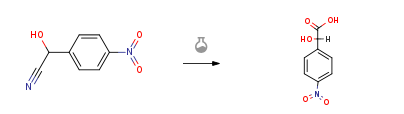
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogenchloride; glacial acetic acid In lithium hydroxide monohydrate at 10 – 20℃; for 6h; | 90% |
| With hydrogenchloride; glacial acetic acid In lithium hydroxide monohydrate at 10 – 20℃; for 6h; | 90% |
| With sulfuric acid | |
| durch Abdampfen mit Salzsaeure; | |
| With hydrogenchloride In glacial acetic acid at 100℃; for 6h; Inert atmosphere; |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 197.147 |
| logP | 0.35 |
| HBA | 3 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 103.35 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| As a pharmaceutical intermediate, it can be used to synthesize various drug molecules. It is often used in pharmaceutical chemistry to produce antibiotics, anticancer drugs and other active pharmaceutical ingredients. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |