4-PHENOXYPHENYLBORONIC ACID CAS#: 51067-38-0; ChemWhat Code: 62230
Identification
| Product Name | 4-PHENOXYPHENYLBORONIC ACID |
| IUPAC Name | (4-phenoxyphenyl)boronic acid |
| Molecular Structure | 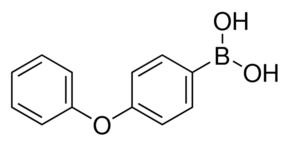 |
| CAS Registry Number | 51067-38-0 |
| EINECS Number | No data available |
| MDL Number | MFCD00093312 |
| Beilstein Registry Number | No data available |
| Synonyms | 4-phenoxyphenylboronic acid, 4-Phenoxyphenylboronic acid CAS#: 51067-38-0 |
| Molecular Formula | C12H11BO3 |
| Molecular Weight | 214.025 |
| InChI | InChI=1S/C12H11BO3/c14-13(15)10-6-8-12(9-7-10)16-11-4-2-1-3-5-11/h1-9,14-15H |
| InChI Key | KFXUHRXGLWUOJT-UHFFFAOYSA-N |
| Canonical SMILES | B(c1ccc(cc1)Oc2ccccc2)(O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110642779 | Diarylpyridine substance and 2,6- preparation method thereof (by machine translation) | 2020 |
| CN110964016 | Aminonorbornene derivative as well as preparation method and application thereof (by machine translation) | 2020 |
| CN110041235 | N – phenyl – N N-P-toluenesulfonyl-trifluoroacetamide and application thereof (by machine translation) | 2019 |
| JP6120272 | A boronic acid group in the auxin biosynthesis inhibitor (by machine translation) | 2017 |
Physical Data
| Appearance | White to off-white solid |
| Flash Point | 377.0±44.0 °C(Predicted) |
| Melting Point, °C | Solvent (Melting Point) |
| 214 – 218 | |
| 115 | |
| 134 – 135 | |
| 123 – 124 | aq. ethanol |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 200 |
| 1H | dimethylsulfoxide-d6 | 200 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene at 90℃; for 3h; Suzuki Coupling; | 96% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene at 90℃; Suzuki Coupling; Inert atmosphere; | 96% |
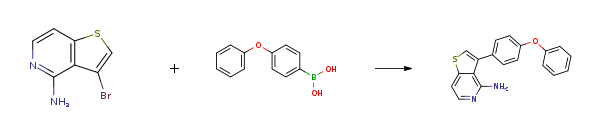
| Stage #1: 3-bromo-4-amino-[3,2-c]-thienopyridine; 4-phenoxyphenylboronic acid With tetrakis(triphenylphosphine) palladium(0) In toluene for 0.0833333h; Inert atmosphere; Stage #2: With sodium carbonate In ethanol; water; toluene at 80℃; for 2h; Inert atmosphere; | 96% |
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In ethanol; water; toluene at 90℃; | 82% |
| With sodium carbonate; Pd(PPh3)4 In ethanol; water; ethyl acetate; toluene; | 82% |
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In DMF (N,N-dimethyl-formamide); water at 80℃; for 18h; | 75% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (16.67%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (83.33%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293190 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 214.029 |
| logP | 2.528 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 49.69 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Target | Effect |
| 8 | Ki (inhibition constant) | < | 10 | nM | Fatty-acid amide hydrolase 1:Wild | |
| 8 | Ki (inhibition constant) | <= | 0.01 | µM | Fatty-acid amide hydrolase 1 [human]:Wild | inhibitory activity |
| 5.28 | inhibition rate | 108 | % | Penicillin-Binding Protein PBP2x [Streptococcus pneumoniae]:Wild | ||
| 5.22 | Ki (inhibition constant) | = | 6 | µM | Carbonic anhydrase 1 [human]:Wild | |
| 4.96 | Ki (inhibition constant) | = | 11 | μM | Carbonic anhydrase 12 [human]:Wild | |
| 3.66 | inhibition rate | 82 | % | Penicillin-Binding Protein PBP2x [Streptococcus pneumoniae]:Wild | ||
| 1 | inhibition rate | 2 | % | D-alanyl-D-alanine carboxypeptidase [Actinomadura]:Wild |
| Use Pattern |
| Commonly used as an intermediate for Ibrutinib. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |