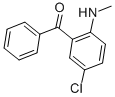
5-Chloro-2-(methylamino)benzophenone CAS#: 1022-13-5; ChemWhat Code: 62757
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117229206 | Preparation method for synthesizing polysubstituted 2-quinolinone compound through base catalysis | 2023 |
| US6407111 | Phenyl substituted pyridine and benzene derivatives | 2002 |
Physical Data
| Appearance | Yellow crystalline powder |
| Melting Point, °C |
| 89 – 91 |
| 93 – 94 |
| 94.2 – 95.4 |
| 94.3 – 95.4 |
| 94.3 – 95.2 |
| 94.2 – 95.3 |
| 94.2 – 95.1 |
| Density, g·cm-3 |
| 1.385 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 600 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 150 |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Spectrum | 13C | chloroform-d1 | 150 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 101 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| ATR (attenuated total reflectance), Bands | |
| Bands | neat (no solvent, solid phase), sodium chloride |
| Intensity of IR bands, Bands | potassium bromide |
| Bands | |
| Bands | potassium bromide |
| Bands | KBr |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Oscillator strength, Band assignment, Spectrum | ethanol | 406 | ||
| ethanol | 532 | |||
| Absorption maxima | methanol | 235, 400 | 20300, 6410 | |
| Spectrum | acetonitrile | 210 – 470 nm | ||
| Absorption maxima | acetonitrile | 236 | 19800 | |
| Absorption maxima | 412 | 6950 | ||
Route of Synthesis (ROS)
| Conditions | Yield |
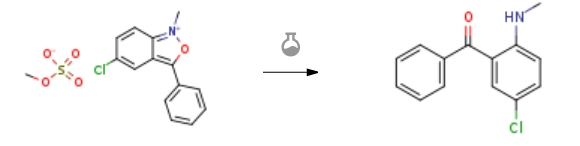
| With hydrazine hydrate; zinc(II) chloride In water at 20 – 30℃; for 4h; Temperature; Experimental Procedure 3 Example 3: Add to the reaction bottleAqueous solution of N-methyl-3-phenyl-5-chloro-2,1-benzisoxazole methyl quaternary ammonium salt 500g(containing N-methyl-3-phenyl-5-chloro-2,1-benzisoxazole methyl quaternary ammonium salt 200g),3 g of zinc chloride and 90 g of an aqueous solution of hydrazine hydrate.The reaction was carried out at 20 to 30 ° C for 3 hours. Cool down, filter,Wash the filter cake with 200g of purified water and dry it.2-methylamino-5-chlorobenzophenone 135g,The yield was 97.7%, the melting point was 94.3 to 95.2 ° C, and the HPLC purity was 99.2%. | 97.7% |
| With hydrazine hydrate; methylamine In water at 25 – 30℃; for 5h; Temperature; Experimental Procedure 3 Example 3: 500 g of an aqueous solution of N-methyl-3-phenyl-5-chloro-2,1-benzisoxazole methyl quaternary ammonium salt was added to the reaction flask (containingN-methyl-3-phenyl-5-chloro-2,1-benzisoxazole methyl quaternary ammonium salt 200 g), 2.4 g of monomethylamine aqueous solution and 60 g of aqueous hydrazine hydrate solution. The reaction was carried out at 25 to 30 ° C for 5 hours. The mixture was cooled, filtered, and washed with 200 g of purified water, and dried to obtain 135 g of 2-methylamino-5-chlorobenzophenone, the yield was 97.7%, the melting point was 94.3 to 95.1 ° C, and the HPLC purity was 99.3%. | 97.7% |
| With Saccharomyces cerevisiae bakers’ yeast In methanol at 20℃; for 8h; pH=7; aq. phosphate buffer; Microbiological reaction; shaking; | 92% |
| With aluminium trichloride; zinc In tetrahydrofuran for 8h; Ambient temperature; | 62% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (97.8%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (97.8%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (95.6%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 245.708 |
| logP | 4.273 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 29.1 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2-Methylamino-5-chlorobenzophenone is an essential intermediate primarily used in synthesizing diazepam and medazepam. These medications are widely used for their anxiolytic, sedative, and anticonvulsant properties. The structural characteristics of 2-Methylamino-5-chlorobenzophenone make it a valuable precursor for synthesizing various pharmacologically active compounds, especially in the benzodiazepine drug class. In diazepam synthesis, 2-Methylamino-5-chlorobenzophenone plays a crucial role by providing the necessary amino and chloroaromatic groups required for the cyclization process, forming the core structure of the medication. Medazepam, another benzodiazepine derivative, has similar sedative effects and is structurally related to diazepam. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |