Benzyl alcohol CAS#: 100-51-6; ChemWhat Code: 102818
Identification
| Product Name | Benzyl alcohol |
| IUPAC Name | phenylmethanol |
| Molecular Structure | |
| CAS Registry Number | 100-51-6 |
| MDL Number | MFCD00004599 |
| Beilstein/REAXYS Number | 878307 |
| EC Number | 202-859-9 |
| eCl@ss | 39023110 |
| Synonyms | benzyl alcohol, benzylic alcohol, Benzylalcohol |
| Molecular Formula | C6H5CH2OH |
| Molecular Weight | 108.14 |
| InChI | InChI=1S/C7H8O/c8-6-7-4-2-1-3-5-7/h1-5,8H,6H2 |
| InChI Key | WVDDGKGOMKODPV-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC=C(C=C1)CO |
Physical Data
| Appearance | Colorless transparent liquid |
| Water Solubility | 33 g/l at 20 °C |
| Flash Point | 96 °C |
| Solubility | water: soluble 33 g/L at 20 °C |
| Melting Point, °C |
| 203 – 205 |
| 204 – 205 |
| -15 |
| 120 – 121 |
| -15.4 |
| -15.19 |
| -15.3 – 0 |
| -15.5 |
| -15.3 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 205.1 | 760 |
| 195 – 200 | 760 |
| 130 – 140 | 6.37564 |
| 92 – 94 | 10 |
| 47 – 48 | 2 |
| 94 – 97 | 12 |
| 80 – 85 | 5 |
| 78 – 79 | 2.5 |
| 50 – 60 | 0.5 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.5831 | 589 | 24.99 |
| 1.53421 | 589 | 34.99 |
| 1.5342 | 589 | 20 |
| 1.53847 | 589 | 25 |
| 1.4018 | 589 | 60 |
| 1.5396 | 589 | 13.6 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.0421 | 24.99 |
| 1.02912 | 39.99 |
| 1.0225 | 49.99 |
| 1.04129 | 25 |
| 1.0401 – 1.0206 | 25 – 50 |
| 1.061 – 0.81 | 0 – 280 |
| 0.9918 – 1.0706 | -15 – 95 |
| 1.214 | -195 |
| Description (Adsorption (MCS)) | Solvent (Adsorption (MCS)) | Temperature (Adsorption (MCS)), °C | Partner (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| Adsorption isotherm | heptane | 24.85 | cellulose | |
| Adsorption | 45 – 55 | quartz sand | ||
| Adsorption | methanol, H2O | 27 | Ratio of solvents: 1:1 v/v, concentration dependence | 2-phenylethanol, 2-methyl-benzyl alcohol |
| Adsorption | methanol, H2O | 27 | Ratio of solvents: 1:1 v/v, concentration dependence | C18-silica gel |
| Desorption | 26.9 – 626.9 | ZnO, clean (0001)-Zn surface | ||
| Desorption | hexane, diethyl ether | Ratio of solvents: 1:1 | graphitized carbon black |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 600 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | 151 |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 300 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | 75 |
| Chemical shifts | 1H | chloroform-d1 | -30 | 300 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Spectrum | various solvent(s) | 80 | pressure dependence |
| Spectrum | liquid carbon dioxide | 80 | high pressure. Object(s) of Study: pressure dependence |
| Bands | neat (no solvent) | 3333 – 1496 cm**(-1) | |
| Bands | KBr | 3300 – 1460 cm**(-1) | |
| Spectrum | neat (no solvent) | 4000 – 666 cm**(-1) | |
| Intensity of IR bands | Intensitaet von OH-Valenzschwingungsbanden bei 3636.3 cmE-1 und 3617.1 cmE-1 von nicht-assoziiertem Benzylalkohol (CCl4). |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | H2O | 250 – 550 nm | |
| Spectrum | cyclohexane | 220 – 280 nm | |
| Spectrum | methanol | 220 – 290 nm | |
| Absorption maxima | cyclohexane | 254.7 | |
| Spectrum | LiCl | 230 – 280 nm, sowie unverduennt. | |
| Absorption maxima | bei -180grad sowie bei +30grad. | 260 |
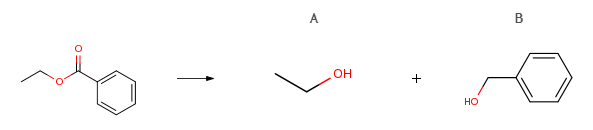
Route of Synthesis (ROS)
| Conditions | Yield |
| With C18H28Br2N4Ru; potassium tert-butylate; hydrogen In 1,4-dioxane at 105℃under 22502.3 Torrfor 8h; | A n/a B 94% |
| With C30H37ClN4ORu; hydrogen; sodium t-butanolate In toluene at 105℃under 4500.45 Torrfor 20hReagent/catalystGloveboxSealed tubeOverall yield = 98 percent; Experimental Procedure General procedure: To a mixture of catalyst (0.01 mmol), KOtBu (10 mol %), and 1,4-dioxane (4.0 mL) in a Parr high-pressure reactor was added the ester(1.0 mmol). The dark red solution was purged with H2 and stirred under 400 psi of H2 at 105 °C for 8 h. Products isolation were performed via column chromatography using silica gel as stationary phase and n-pentane/ethylacetate or n-pentane/isopropanol mixture as eluent. The products were confirmed by 1H NMR. | A n/a B 92% |
| With C42H38N4OPRu(1+)*Cl(1-); potassium tert-butylate; hydrogen In toluene at 100℃under 4104.28Torrfor 2h; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] |
| Precautionary Statement Codes | P261, P264, P270, P271, P301+P312, P304+P312, P304+P340, P312, P330, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous good |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 2.1/kg |
| Use Pattern |
| Benzyl alcohol CAS#: 100-51-6 can be used in cosmetics/dental/toilet |
| inhibiting bacteria |
| a cosmetic or dermatological composition, in combination with 4-(3-ethoxy-4-hydroxyphenyl)butan-2-one |
| natural broad-spectrum preservative composition in combination with extract of citrus grandis, fruit, octanohydroxamic acid and polyol |
| a sensate in a personal care composition, in combination with 2-isopropyl-N,2,3-trimethylbutyramide |
| Benzyl alcohol CAS#: 100-51-6 is a sensate in a personal care composition, in combination with camphor |
| sensate in afier shaves lotions |
| preservative in pharmaceutical compositions comprising kisspeptin or derivatives thereof, used in synchronization programs of production animal estrus |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watson Noke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |