Dapagliflozin CAS#: 461432-26-8; ChemWhat Code: 17054
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN115785045 | Photocatalytic Endagliflozin precursor and synthesis method thereof | 2023 |
| WO2021/105152 | USE OF SGLT-2 INHIBITORS IN THE DRYING-OFF OF NON-HUMAN MAMMALS | 2021 |
| CN108285439 | Carbon glucoside sodium glucose transport protein body 2 inhibitor | 2018 |
| CN108675976 | 6-halogenated glucose C-glycoside as well as preparation method and application thereof | 2018 |
Physical Data
| Appearance | white to grayish white powder |
| Melting Point, °C | Solvent (Melting Point) |
| 87 – 89 | |
| 88.3 | ethyl acetate, n-heptane |
| 88 – 89 | |
| 55.08 – 58.63 |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | 21.84 | bovine serum albumin |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | |||
| Chemical shifts, Spectrum | 13C | |||
| Chemical shifts, Spectrum | 1H | 25 | ||
| Chemical shifts, Spectrum | 13C | 25 | ||
| Chemical shifts, Spectrum | 1H | |||
| Chemical shifts | 1H | [D3]acetonitrile | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | sodium chloride |
| Bands, Spectrum | potassium bromide |
| Bands | methanol |
| Bands, Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
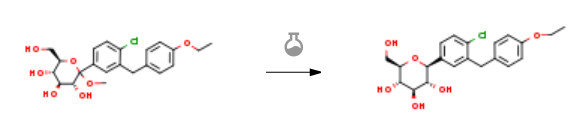
| With triethylsilane; boron trifluoride diethyl etherate In methanol; dichloromethane; ethyl acetate at -20 – 20℃; for 5h; Experimental Procedure Compound 4a’ (10.0 g, 22.1 mmol, 1.0 eq, purity 90.2%) was dissolved in dichloromethane (50 mL) and EtOAc (50 mL).The above solution was added to the reaction flask, and triethylsilane (7.7 g, 66.2 mmol, 3.0 eq) was added under nitrogen atmosphere, and the temperature was lowered to -20 ° C to -10 ° C, and boron trifluoride etherate (6.3 g, 44.2) was added dropwise. Methanol, 2.0 eq), after completion of the dropwise addition, the mixture was stirred for 4 hours, and then further stirred at room temperature for 1 hour. Saturated sodium hydrogen carbonate solution (100 mL) and ethyl acetate (100 mL) were added to the reaction mixture, and the mixture was separated, and the aqueous phase was extracted again with ethyl acetate (50 mL). The organic phase was combined and washed with water (50 mL) The aqueous sodium sulfate was dried, filtered and concentrated to dryness to give 7.4 g of the compound of formula I, yield 83.1%, purity 85.0%. | 83.1% |
| Stage #1: (3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol With triethylsilane; boron trifluoride diethyl etherate In dichloromethane; acetonitrile at -10 – 20℃; Stage #2: With water; sodium hydrogencarbonate In dichloromethane; acetonitrile Experimental Procedure (2S,3R,4R,5S,6R)-2-(3-(4-Ethoxybenzyl)-4-chlorophenyl)-6- (hydroxymethyl)-tetrahydro-2H-pyran-3A5-triol: At about -10 0C, triethylsilane (343 mg, 2.96 mmol, 3.00 equiv) and boron trifluoride diethyl etherate (420 mg, 2.96 mmol, 3.00 equiv) were added dropwise to a stirred solution of (3R,4S,5S,6R)-2-(3-(4- ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-2-methoxy-tetrahydro-2H-pyran- 3,4,5-triol (300 mg, 0.68 mmol, 1.00 equiv) and acetonitrile / dichloromethane (50 niL). After stirring at ambient temperature for about 16 hours, the reaction was quenched by adding a saturated solution of sodium bicarbonate (50 mL). Standard extractive workup with ethyl acetate (3 x 200 mL) gave a crude residue which was first purified by silica gel column chromatography (eluted with dichloromethane / methanol (20 : 1) and then purified by preparative ΗPLC to remove the (2R,3R,4R,5S,6R)-2-(3-(4- ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol isomer. The title product was isolated as a white solid (130 mg; yield = 44%). 1H NMR (300 MHz, CDC13), δ: 7.37 (d, J = 8.1 Hz, IH), 7.32 (d, J = 1.8 Hz, IH), 7.24 (dd, J= 8.1, 1.8 Hz, IH), 7.09 (d, J= 8.4 Hz, IH), 6.82 (d, J= 8.4 Hz, IH), 4.83-5.00 (br m, 3H), 4.44 (s, IH), 3.93-4.03 (m, 5H), 3.67-3.71 (m, IH), 3.10-3.47 (m, 6H), 1.30 (t, J= 7.2 Hz, 3H); LC-MS : m/z= 453 (M+HCOO) “. | 44% |
Safety and Hazards
| Pictogram(s) | |
| Signal | Danger |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H318 (28.57%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (14.29%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H372 (57.14%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] H373 (28.57%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H411 (14.29%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] H413 (28.57%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P260, P264, P264+P265, P270, P273, P280, P301+P317, P305+P351+P338, P305+P354+P338, P317, P319, P330, P337+P317, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 408.879 |
| logP | 2.937 |
| HBA | 5 |
| HBD | 4 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 99.38 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Dapagliflozin is a sodium-glucose co-transporter 2 inhibitor (SGLT2i) and a new type of oral hypoglycemic drug. It can inhibit SGLT2 activity, inhibit proximal renal tubular sodium-glucose reabsorption, promote urinary glucose excretion, and thereby reduce blood glucose concentration. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |