| Conditions | Yield |
| 100% |
In 1,4-dioxane; water
Experimental Procedure
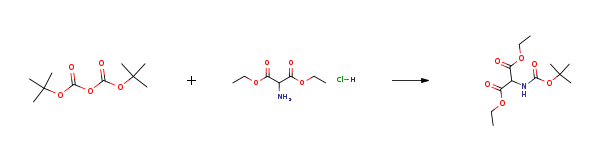
2-tert-Butoxycarbonylamino-3-ethoxy-3-oxopropanoic acid (6)
To a suspended of diethyl aminomalonate hydrochloride (25 g, 118.12 mmol, 1 equiv) in a mixture of water (150 mL) anddioxane (220 mL) in a round bottom flask with magnetic bar, NaHCO3 (10.42 g, 124.03 mmol, 1.05 equiv) was slowlyadded while stirring at room temperature. When the solution became clear, a catalyst amount of DMAP (1% mol, 144 mg)was added followed with a dropwise addition of a solution of Boc2O (27.07 g, 124.03 mmol, 1.05 equiv) in dioxane (80mL). After the reaction was complete (monitored by TLC), the solvents were evaporated in vacuo. The residue wasdissolved in EtOAc. The organic phase was washed with solutions of 5% KHSO4, satd. NaHCO3, water, and brine, anddried over anhydrous Na2SO4, then filtered and evaporated in vacuo. The desired product was pure enough for the nextstep without a need of purification via column chromatography. Yield quantitative (32.51 g). 1H NMR (400 MHz,DMSO-d6): δ 7.67 (d, J = 8.1 Hz, 1H), 4.80 (d, J = 8.1 Hz, 1H), 4.16 (m, 4H), 1.39 (s, 9H), 1.20 (t, J = 7.1 Hz, 6H). 13CNMR (100 MHz, DMSO-d6): δ 167.04, 155.54, 79.51, 61.96, 57.89, 28.48, 12.28. HRMS (ESI, positive mode): m/z298.1323 [M+Na]+, calcd for [C12H21NO6Na]+: 298.1261 | 100% |
With triethylamine In tetrahydrofuran; water at 0 – 55℃; for 50h;
Experimental Procedure
100.a a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67)
a) 1,3-diethyl 2-{ r(fe/t-butoxy)carbonyl1 amino jpropanedioate (T67) Di-ie/t-butyl dicarbonate (5.3 g; 24 mmol; 1 eq) and triethylamine (3 mL) at 0°C were added to a solution of 1,3-diethyl 2-aminopropanedioate hydrochloride (5 g; 23 mmol; 1 eq) in tetrahydrofuran/water (1: 1, 60 mL). The reaction mixture was stirred at room temperature for 2 days and, at 55°C, 2 hours. After concentration to dryness, the residue was taken up in ethyl acetate (150 mL) and water (50 mL). The aqueous layer was extracted with ethyl acetate (2 x 50 mL). The combined organic layers were washed with saturated ammonium chloride (50 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The title compound, 1,3-diethyl 2- { [(ieri-butoxy)carbonyl] amino Jpropanedioate was obtained in 97% yield (6.16 g) as a colorless oil. 1H NMR (CDC13): δ (ppm) 1.35 (t, 6H), 1.5 (s, 9H), 4.32 (q, 4H), 4.99 (d, 1H), 5.61 (d, 1H). | 97% |
| With triethylamine In 1,4-dioxane; water at 0 – 55℃; for 15.25h; | 96% |
| With sodium hydrogencarbonate In dichloromethane for 3h; Heating; | 95% |
With triethylamine In ethanol at 0 – 5℃; for 3.5h; Inert atmosphere;
Experimental Procedure
1.2 1.2 Diethyl 2-(ferf-butoxycarbonyl)amidomalonate
Diethyl 2-aminomalonate hydrochloride (2.535 g, 12.0 mmol) was dissolved in a mixture of 1 M NaOH (12 mL) and 1 ,4-dioxane (10 mL) and a solution of Boc-anhydride (2.54 g, 12.0 mmol, 1.0 eq.) in 1 ,4-dioxane (5 mL) was added dropwise at 5 °C. Subsequently, the mixture was stirred at r.t. for 24 h. Dioxane was removed in vacuo and the residue was dissolved in ethyl acetate. After phase separation, the organic layer was washed with 1 M HCI (3 x 50 mL) and dried over Na2S04. The solvent was removed in vacuo and the crude product was purified by column chromatography with silica gel (cyclohexane/ethyl acetate, 6:1 ). The product was isolated as a colourless oil. Yield: 3.009 g (91 %). 2?1H NMR (300 MHz, CDCI3): δ [ppm]: 1 .30 (t,?3JKH?= 7.2 Hz, 6 H, 10-CH3, 12-CH3), 1 .45 (s, 9 H, 6-CH3, 7-CH3, 8-CH3), 4.27 (m, 4 H, 9-CH2, 1 1 -CH2), 4.94 (d,?3JH,H?= 7.7 Hz, 1 H, 2-CH), 5.63 (d,?3JH,H = 7.8 Hz, 1 H, 2-NH).?13C-NMR (101 MHz, CDCI3) δ [ppm]: 14.0 (q, C-10, C-12), 28.2 (q, C-6, C-7, C-8), 57.5 (d, C-2), 62.4 (t, C-9, C-1 1 ), 80.5 (s, C-5), 154.8 (s, C-4), 166.6 (s, C-1 , C-3). Exact mass (ESI+): Ci2H2iN06?+ Na+: calcd. 298.1261 , found 298.1244. Ref.:?1H NMR: H. Schneider, G. Sigmund, B. Schricker, K. Thirring, H. Berner, J.Org. Chem. 1993, 58, 683-689. | 94.6% |
| With sodium hydroxide In 1,4-dioxane at 5 – 20℃; for 24h; | 91% |