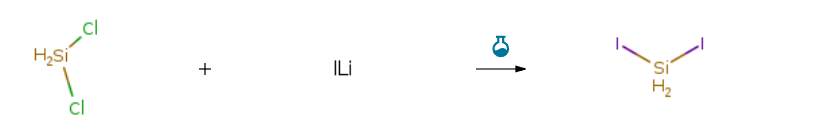Diiodosilane CAS#: 13760-02-6; ChemWhat Code: 1415106
Identification
| Product Name | Diiodosilane |
| Molecular Structure | 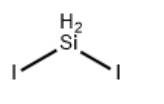 |
| CAS Registry Number | 13760-02-6 |
| MDL Number | MFCD00075171 |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 |
| Molecular Formula | I2Si |
| Molecular Weight | 281.894 |
| InChI | InChI=1S/I2Si/c1-3-2 |
| InChI Key | RNRZLEZABHZRSX-UHFFFAOYSA-N |
| Isomeric SMILES | [Si](I)I |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2023/522969 | Method for preparing iodosilane and composition thereof | 2023 |
| WO2017/201456 | PREPARATION OF SI-H CONTAINING IODOSILANES VIA HALIDE EXCHANGE REACTION | 2017 |
Physical Data
| Appearance | Colorless to Yellow to Orange to Pink to Faint Red Liquid |
| Melting Point, °C | Solvent (Melting Point) |
| -1 |
| Boiling Point, °C |
| 149.5 |
| 150 |
| Density, g·cm-3 | Reference Temperature, °C |
| 2.729 | 20.5 |
| 2.73 | 20 |
| 2.745 | 15.5 |
| 2.746 | 15.1 |
| 2.7605 | 10.8 |
| 2.772 | 7 |
| 2.777 | 5.1 |
Spectra
No data available
Route of Synthesis (ROS)
Route of Synthesis (ROS) of CAS Diiodosilane CAS 13760-02-6
| Conditions | Yield |
| In pentane at -70 – 35℃; for 16h; Temperature; Solvent; Inert atmosphere; Large scale; | 77% |
| at 40℃; under 1277.21 – 2311.54 Torr; for 0.08h; Temperature; Experimental Procedure This example demonstrates a method of the invention in which dichlorosilane is converted to diiodosilane. (0079) [0034] A jacketed vertical stainless-steel tube, measuring 29 inches long with a diameter of 0.5 inches, was plumbed from the bottom outlet to a glass round bottom flask with a skin temperature maintained at 100 °C. The flask was equipped with a tap water condenser which was vented to a dry ice cooled stainless steel canister. The tube jacket was maintained at 40 °C by recirculating a temperature controlled fluid. The system was purged with nitrogen and the tube was loaded with 80 g of anhydrous lithium iodide. 402 g of dichlorosilane was fed to the top end of the tube at such a rate as to achieve a residence time inside of the tube of 4.8 minutes while maintaining a back pressure of 10-30 psig. Once the internal temperature of the eluent flask was within 5 °C of the skin temperature the mixture was transferred to a distillation apparatus consisting of a round bottom flask equipped with a magnetic stirrer, and fitted with an 8 inch column packed with glass packing. The distillation column was equipped with a tap water condenser. The pressure of the distillation system was reduced to 30 mmHg and the pot was heated until the temperature above the column began to rise indicating the complete removal of dichlorosilane. 45.3 g of diiodosilane was isolated from the distillation bottom in 99.7% purity as indicated by GC-TCD. |
Safety and Hazards
No data available
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 283.91 |
| logP | 2.237 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Diiodosilane CAS#: 13760-02-6 can be used as coupling agents, surfactants, and catalysts. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to [email protected] |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |