DIPHENYLCYCLOPROPENONE CAS#: 886-38-4; ChemWhat Code: 1180944
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN114031497 | Ring-opening dichlorination reaction method of cyclopropenone and oxygen heterocyclic compound | 2022 |
| CN113929651 | Method for synthesizing alpha-pyrone compound | 2022 |
| CN113896630 | Ring-opening diiodination reaction method of cyclopropenone and oxygen heterocyclic compound | 2022 |
| CN114573449 | Catalyst for preparing carboxylic acid acyl chloride through phosgenation reaction of carboxylic acid and application of catalyst | 2022 |
| CN112174880 | Preparation method of 1,3,4,6-tetra-substituted pyridinone derivative | 2022 |
| WO2022/188082 | METHOD FOR CARRYING OUT REACTION OF ISATIN COMPOUND AND CYCLOPROPENONE COMPOUND AT LOW CATALYTIC AMOUNT | 2022 |
| WO2022/226855 | METHOD FOR PREPARING PYRANO[2,3-B]-INDOLE-2-ONE WITHOUT CATALYST | 2022 |
Physical Data
| Appearance | Off-white to light cream fine powder |
| Melting Point, °C | Solvent (Melting Point) |
| 120 – 121 | |
| 119 – 120 | |
| 114 – 116 | |
| 121.5 | |
| 87 | |
| 118 – 120 | cyclohexane |
| 120 | aq. ethanol |
| Density, g·cm-3 |
| 1.1 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| NMR spectrum of the complex | D2O, acetone-d6 | 20 | 1-ethoxy-2,3-diphenylcyclopropenium ion, 2.0 M DClO4, 1.5 M LiClO4 |
| Stability constant of the complex with … | |||
| Enthalpy of association | |||
| Spectrum of the complex | |||
| Further physical properties of the complex |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
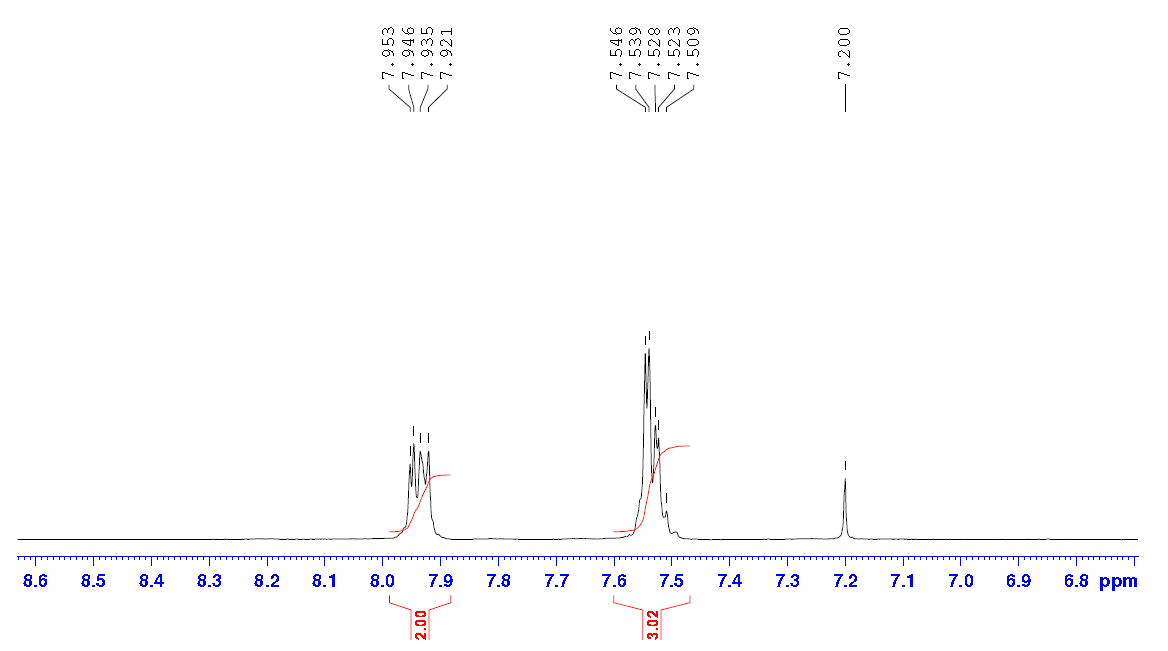
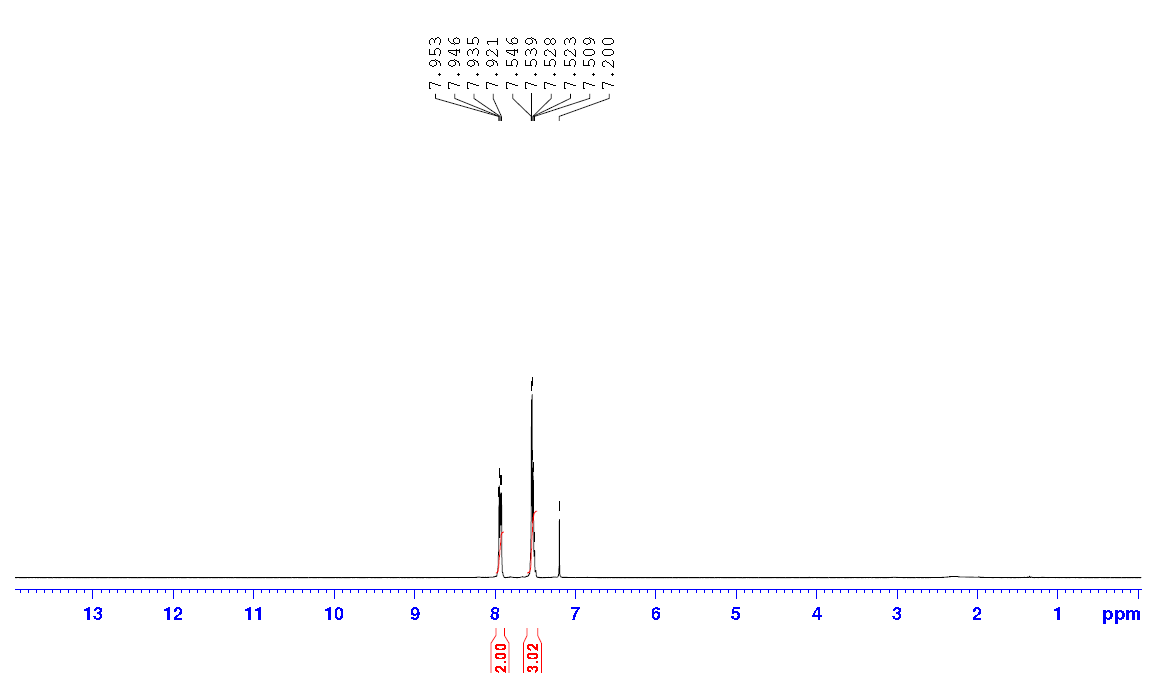
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 100 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 | |
| Spectrum | 13C | chloroform-d1 | ||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 500 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | |
| ATR-FTIR (attenuated total reflectance Fourier transform infrared spectroscopy), Bands | ||
| Bands, Spectrum | dichloromethane | |
| Spectrum | acetonitrile | |
| Spectrum | CCl4 | |
| Spectrum | hexane | |
| Bands | 1850 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | H2 aq. phosphate buffer | ||
| Spectrum | dimethyl sulfoxide | 302 | |
| 345, 325 | |||
| Spectrum | cyclohexane | ||
| Spectrum | methanol | Remark: 25 deg C | |
| methanol | 295 | ||
| CH2Cl2 | 226, 295 |
Route of Synthesis (ROS)
| Conditions | Yield |
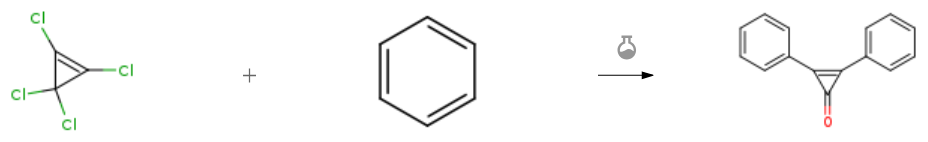
| Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In dichloromethane at -78 – 20℃; for 4h; Inert atmosphere; Sealed tube; Stage #2: With water In dichloromethane Inert atmosphere; Sealed tube; | 85% |
| Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In dichloromethane for 0.5h; Stage #2: With water; sodium hydroxide In dichloromethane at 20℃; for 1.5h; Experimental Procedure 4.2. General procedure for the synthesis of cyclopropenones General procedure: The AlCl 3 (0.67 g, 5 mmol) was added by portions to a solu- tion of one of the compounds 2a-d (25 mmol) and tetrachloro- cyclopropene (12.5 mmol) in anhydrous CH 2 Cl 2 (200 mL) un- der continuous stirring for 30 min. The stirring was continued for another 90 min at room temperature, then the mixture was poured in cold water (200 mL). The organic layer was sepa- rated, washed with water (2 ×50 mL) and dried with MgSO 4 . After the solvent was distilled offin vacuo , the residue was chromatographed on Al 2 O 3 using a hexane-CH 2 Cl 2 (3:1) mix- ture as eluent to afford compounds 2,3-diphenylcyclopropenone 3a , yield, 1.64 g, 7.8 mmol (63%), m.p. 120-121 °C (lit.: m.p. 121-121.5 °C [32] ), 2,3- bis (4-methoxyphenyl)cyclopropenone 3b , yield, 2.23 g, 8.4 mmol (67%), m.p. 153-154 °C (lit.: m.p.153-155 °C [33] ), 2,3-diferrocenyl-cyclopropenone 3c , yield, 4.5 g, 10.6 mmol (85.3%), m.p. 182-184 °C (lit.: m.p. 182-183 °C [20] ), 2,3-diruthenocenylcyclopropenone 3d , yield, 3.8 g, 7.5 mmol (60%), m.p. 256-258 °C (lit.: m.p. 256-257 °C | 63% |
| With aluminium trichloride; water 1.) 1,2-dichloroethane, room temp.; | |
| Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In carbon disulfide; dichloromethane at -78 – 20℃; Inert atmosphere; Stage #2: With water In carbon disulfide; dichloromethane Inert atmosphere; Experimental Procedure 2,3-Diphenylcyclopropenone (8b) [1,3]. In a 100 mL one-necked round-bottomed flask fitted with a septum, 1.6 mmol (0.285 g) of tetrachlorocyclopropene and 3.38 mmol (0.45 g) of aluminum chloride (AlCl3) were placed and 25 mL of dichloromethane (freshly dried over NaH) were added. The flask was cooled in an acetone/dry ice bath to 78 °C and stirred with a magnetic stirrer under argon atmosphere. A mixture of 3.2 mmol (0.25 g) of benzene and 2.5 mL of dry CH2Cl2 was added dropwise to the reaction mixture. After the benzene solution was completely dropped in, the mixture was magnetically stirred at 78 °C for 2 h and then allowed to warm to room temperature. The mixture was further stirred for 24 h and after that time, a portion of water was added, and the resulting solution was extracted twice with CH2Cl2. The organic layer was separated and washed with water and brine. The solvent was evaporated and the crude product, after recording the 1H NMR spectrum, was purified on a SiO2 column using a mixture of petroleum ether and ethyl acetate (ratio 4:1) as the eluent. The major fraction containing crude 8b was separated in 74% yield as pale-brown crystals. The product was not purified but used directly as obtained for the synthesis of thioketone 6b; therefore, the calculated yields of both 8b and 6b are unreliable. Pale-brown crystals; m.p. 119-121 °C (after washing with PE) (ref. [1], m.p. 120-121 °C). 1H NMR (600 MHz, CDCl3): d 7.70-7.73 (m, 6CHarom); 7.79-8.05 (m, 4CHarom). | |
| Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In dichloromethane Friedel-Crafts Alkylation; Stage #2: With sodium hydroxide In water Friedel-Crafts Alkylation; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (85.7%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (98%): May cause an allergic skin reaction [Warning Sensitization, Skin] |
| Precautionary Statement Codes | P261, P264, P272, P280, P302+P352, P321, P332+P317, P333+P317, P362+P364, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 206.244 |
| logP | 3.602 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 17.07 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| DIPHENYLCYCLOPROPENONE CAS#: 886-38-4 increasing peripheral blood mononuclear cell (PBMC) expression of interferon gamma in response to a viral or fungal immune stimulus in a person, infection of the skin or mucous membrane or causes lesions on the skin or mucous membrane,and increasing PBMC interferon gamma expression in response to a viral or fungal immune stimulus. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | http://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |