Hexadecyl trimethyl ammonium bromide CAS#: 57-09-0; ChemWhat Code: 122788
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2023/156758 | FUNGICIDES AND USES THEREOF | 2023 |
| US2020/163990 | NOVEL BACTERICIDES AND ANTIFUNGAL AGENTS | 2020 |
| CN111620786 | Active bromine quaternary ammonium salt as well as preparation method and application thereof | 2020 |
| CN111825586 | Preparation method of xanthate compound | 2020 |
| US2013/75670 | AMINOBENZENE COMPOSITIONS AND RELATED DEVICES AND METHODS | 2013 |
Physical Data
| Appearance | White to almost white powder |
| Melting Point, °C | Solvent (Melting Point) |
| 240 – 242 | |
| 242 | |
| 247 | |
| 248 – 251 | |
| 247 | |
| 207 – 208 | |
| 237 – 243 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.167 | -100.16 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Adsorption isotherm | Q235 mild steel | ||
| Rate of adsorption | Q235 mild steel | ||
| Adsorption and desorption isotherms | silica | ||
| Adsorption isotherm | water | 19.84 | clinoptilolite |
| Adsorption isotherm | water | 39.84 | clinoptilolite |
| Adsorption isotherm | water | 4.84 | clinoptilolite |
| Enthalpy of adsorption | water | 19.84 | clinoptilolite |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts | 1H | water-d2 | 18.84 | |
| Chemical shifts | 1H | water-d2 | ||
| Chemical shifts, Spectrum | 1H | water-d2 | 500 | |
| Chemical shifts, Spectrum | 13C | water-d2 | 126 | |
| Spectrum | 1H | water-d2 | 25 | |
| Spectrum | 1H | water-d2 | 45 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | potassium bromide |
| Bands, Spectrum | |
| Spectrum | |
| Bands, Spectrum | neat (no solvent, solid phase) |
| ATR (attenuated total reflectance), Spectrum | |
| Spectrum | |
| ATR (attenuated total reflectance), Bands, Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | |
| Spectrum | aq. phosphate buffer |
| Spectrum | aq. buffer |
| Spectrum | water |
| Spectrum | |
| Spectrum | sodium chloride, water |
Route of Synthesis (ROS)
| Conditions | Yield |
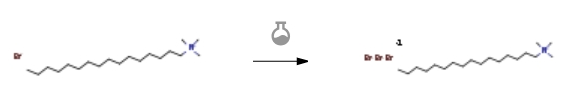
| With dihydrogen peroxide; sodium carbonate; potassium bromide In water at 20℃; for 0.0833333h; Experimental Procedure Procedure for Synthesis of CTMATB CTMATB was synthesised using a modified version of our method reported earlier.17 In this procedure, a mixture of 4.89 g (41.07 mmol) of potassium bromide (KBr) and 5.00 g (13.74 mmol) of cetyltrimethylammonium bromide (CTMAB), and 0.057 g (0.53 mmol) of sodium carbonate (Na2CO3) were taken in a mortar and 10 mL (88.24 mmol) of 50% H2O2 added to the whole. The resultant mixture was grinded thoroughly and then was dissolved in 50 mL of water taken in a 100 mL beaker. The reaction solution was stirred at room temperature for 5 minutes and then 30 mL of 1 M H2SO4 was added drop-wise. An exothermic reaction followed and the CTMATB precipitated out. CTMATB formed was filtered using suction pump, washed with water many times till the filtrate contained no trace of acid (tested using litmus paper), and then initially air-dried and finally dried in a vacuum dessiccator. The compound was then dried in a vacuum desiccator using anhydrous calcium chloride (CaCl2) as desiccant. The product was obtained as bright yellow micro-crystals which was further recrystallized from methanol. Yield of the product was 5.52 g (96%), m.p. 87-88 °C, m.p. (lit.) 87 or 88 °C.35 | 96% |
| With 3-chloro-benzenecarboperoxoic acid; potassium bromide In water for 0.1h; Experimental Procedure 5. Synthesis of cetyltrimethyl ammonium tribromide, CTMTB 5. Synthesis of cetyltrimethyl ammonium tribromide, CTMTB: 1 equiv of cetyltrimethyl ammoniumbromide (1 mmol, 0.364 g), 2 equiv of KBr (2 mmol, 0.238 g) and 2 equiv of MCPBA (2 mmol, 0.346 g)were mixed together in 10 mL of water and stirred for ca. 6 min. The orange coloured product formedwas washed with NaHCO3 solution (10 % solution) for several times to remove unreacted substrate. Afterthat the crude product was again washed with water to remove by-products. The compound was dried invacuo and recrystallized with EtOAc; mp: 90oC. Yield: 0.439 g; 90 %. | 90% |
| With oxone; sodium bromide In water for 1h; Experimental Procedure 2.1-2.4 Preparation of Cetyl Trimethyl Ammonium Tribromide (2b) Step 1. Dissolve 1 g of cetyl trimethyl ammonium bromide in water to prepare a cetyl trimethyl ammonium bromide solution. The mass ratio of the water to cetyl trimethyl ammonium bromide is 80:1;Step 2. Add NaBr to the cetyltrimethylammonium bromide solution and stir, the stirring time is 0.5 hours, the stirring speed is 600rpm, the NaBr and the cetyltrimethyl bromide The molar mass ratio of ammonium hydroxide is 2.5:1;Step 3. Add dropwise an oxone aqueous solution of potassium hydrogen persulfate composite salt (oxone) to the mixture prepared after the completion of step 2, at a stirring speed of 600 rpm, and continue the reaction for 0.5 hours after the addition of the potassium hydrogen persulfate composite salt aqueous solution is completed, to obtain The reaction product, wherein the mass content of the potassium bisulfate composite salt in the potassium hydrogen persulfate composite salt aqueous solution is 20% of the molar mass ratio of the added potassium hydrogen persulfate composite salt to the cetyltrimethylammonium bromide 1.5:1;In step 4, the reaction product finally obtained in step 3 is filtered to obtain a filter cake. The filter cake is ultrasonically recrystallized with 1 times the mass of acetonitrile for 1 min to obtain cetyltrimethylammonium tribromide with a yield of 90%. | 90% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H302 (96.9%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (44.3%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (91.6%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H335 (37.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H373 (33.4%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H400 (95%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (24.5%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P260, P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P305+P354+P338, P317, P319, P321, P330, P332+P317, P362+P364, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| HS Code | |
| Storage | Stored at room temperature and keep dry and light; Sealed and keep ventilation. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 364.453 |
| logP | 8.9 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 15 |
| Matching Veber Rules | 1 |
| Use Pattern |
| As an ingredient to adjust the surface properties of cosmetic systems, it is used in cleansing products (such as cleansers and shampoos). Antimicrobial preservatives Due to its cationic properties, CTAB has an inhibitory effect on bacteria and certain fungi and can be used as a certain antimicrobial preservative ingredient. Emulsifiers and dispersants |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |