Imidodisulfuryl fluoride lithium salt CAS#: 171611-11-3; ChemWhat Code: 557448
Identification
| Product Name | Imidodisulfuryl fluoride lithium salt |
| IUPAC Name | lithium;bis(fluorosulfonyl)azanide |
| Molecular Structure | 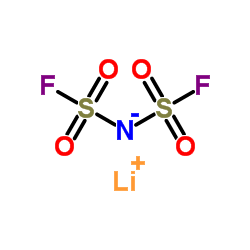 |
| CAS Registry Number | 171611-11-3 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | ithium bis(fluorosulfonyl)imideLiFSIlithium bis(fluorosulfonyl)amidelithium di(fluorosulfonyl)imidebis(fluorosulfonyl)imide lithium salt |
| Molecular Formula | F2LiNO4S2 |
| Molecular Weight | 187.072 |
| InChI | InChI=1S/F2NO4S2.Li/c1-8(4,5)3-9(2,6)7;/q-1;+1 |
| InChI Key | VDVLPSWVDYJFRW-UHFFFAOYSA-N |
| Canonical SMILES | [Li]N(S(=O)(=O)F)S(=O)(=O)F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2023/25776 | REACTIVE DISTILLATION PROCESS FOR PREPARING FLUOROSULFONYLIMIDE SALTS | 2023 |
| CN117209535 | Synthesis method and application of bis (fluorosulfonyl) imine trialkyl phosphine salt | 2023 |
Physical Data
| Appearance | White solid |
| Solubility | Soluble in water |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C |
| 137 |
| 143.5 |
| 124 – 128 |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.14 | 4 | 25 |
| 1.2 | 4 | -190 |
| 1.24 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | CCl4 | 24.9 | 4-Fluorophenol |
| Stability constant of the complex with … | aq. HNO3 | 25 | AgNO3 |
| Enthalpy of association | acetonitrile | 25 | iodine |
| NMR spectrum of the complex | CDCl3 | Cu(2,4-dichloro-benzoate)2 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Spectrum | 7Li | |
| Spectrum | 19F | 20 |
| Spectrum | 19F | 30 |
| Spectrum | 19F | 40 |
| Description (IR Spectroscopy) | Original Text (IR Spectroscopy) | Comment (IR Spectroscopy) | |
| Bands, Spectrum | |||
| Bands | potassium bromide | ||
| Bands, Spectrum | As measured using Fourier Infrared Spectrometer, 1401cm-1, 1386cm-1, 1190cm-1, 1225cm-1, 859cm-1, 845cm-1, 783cm-1, 747cm-1 | ||
| Spectrum | neat (no solvent, solid phase) | 200 cm**-1 – 4000 cm**-1 |
| Description (Raman Spectroscopy) |
| Spectrum, Bands |
| Spectrum |
| Bands |
| Raman |
Route of Synthesis (ROS)
| No data available |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H301 (66.7%): Toxic if swallowed [Danger Acute toxicity, oral] H302 (33.3%): Harmful if swallowed [Warning Acute toxicity, oral] H314 (66.7%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H315 (33.3%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (33.3%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (100%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H361 (33.3%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H412 (66.7%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P203, P260, P261, P264, P264+P265, P270, P272, P273, P280, P301+P316, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P318, P321, P330, P332+P317, P333+P317, P362+P364, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 187.074 |
| logP | -1.182 |
| HBA | 5 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 85.04 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Lithium Bis(fluorosulfonyl)imide (LiFSI, CAS 171611-11-3) is a widely used electrolyte additive in lithium-ion batteries due to its unique properties. LiFSI has high ionic conductivity and excellent solubility in a variety of organic solvents, contributing to improved overall electrolyte performance. It supports stable operation over a broad voltage range, which is particularly beneficial for high-energy-density batteries. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

