L-Homocystine CAS#: 626-72-2; ChemWhat Code: 82589
Identification
| Product Name | L-Homocystine |
| IUPAC Name | (2S)-2-amino-4-[[(3S)-3-amino-3-carboxypropyl]disulfanyl]butanoic acid |
| Molecular Structure | 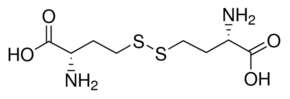 |
| CAS Registry Number | 626-72-2 |
| EINECS Number | 210-962-5 |
| MDL Number | MFCD00020391 |
| Beilstein Registry Number | 1728583 |
| Synonyms | L-homocystine, L-Homocystine, (2S)-2-amino-4-{[(3S)-3-amino-3-carboxypropyl]disulfanyl}butanoic acid, L-4,4′-dithio-bis(2-aminobutanoic acid), (L,L)-homocystine, l-homocystine, homocystine CAS Number: 626-72-2 |
| Molecular Formula | C8H16N2O4S2 |
| Molecular Weight | 268.354 |
| InChI | InChI=1S/C8H16N2O4S2/c9-5(7(11)12)1-3-15-16-4-2-6(10)8(13)14/h5-6H,1-4,9-10H2,(H,11,12)(H,13,14)/t5-,6-/m0/s1 |
| InChI Key | ZTVZLYBCZNMWCF-WDSKDSINSA-N |
| Canonical SMILES | C(CSSCCC(C(=O)O)N)C(C(=O)O)N |
| Isomeric SMILES | C(CSSCC[C@@H](C(=O)O)N)[C@@H](C(=O)O)N |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US9879043 | Synthesis of non-natural cofactor analogs of S-adenosyl-L-methionine using methionine adenosyltransferase | 2018 |
| US2018/305307 | COMPOUNDS AS L-CYSTINE CRYSTALLIZATION INHIBITORS AND USES THEREOF | 2018 |
Physical Data
| Appearance | No data available |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | 1.443±0.06 g/cm3(Predicted) |
| Sensitivity | No data available |
| Melting Point, °C |
| 255 – 265 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Comment (Association (MCS)) | Partner (Association (MCS)) |
| Further physical properties of the complex | acetonitrile, H2O | 24.9 | temperature dependence | poly(4-((1-aza-4,7,10,13,16-pentaoxacyclooctadecan-1-yl)-carbonyl)phenylacetylene) |
| UV/VIS spectrum of the complex | acetonitrile, H2O | poly(4-((1-aza-4,7,10,13,16-pentaoxacyclooctadecan-1-yl)-carbonyl)phenylacetylene) | ||
| NMR spectrum of the complex | CD3CN, H2O | 25 | poly(4-((1-aza-4,7,10,13,16-pentaoxacyclooctadecan-1-yl)-carbonyl)phenylacetylene) | |
| NMR spectrum of the complex | CD3CN, H2O | 45 | poly(4-((1-aza-4,7,10,13,16-pentaoxacyclooctadecan-1-yl)-carbonyl)phenylacetylene) | |
| NMR spectrum of the complex | CD3CN, H2O | 55 | poly(4-((1-aza-4,7,10,13,16-pentaoxacyclooctadecan-1-yl)-carbonyl)phenylacetylene) |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Coupling Nuclei | Solvents (NMR Spectroscopy) |
| Chemical shifts | 1H | D2O, NaOD | |
| 1H | 1H | D2O, NaOD | |
| Chemical shifts | 13C | D2O, NaOD | |
| Chemical shifts | 13C | D2O, NaOD | |
| Spin-lattice relaxation time (T1) | D2O, NaOD |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Spectrum | KBr | 5000 – 1250 cm**(-1) |
| Spectrum | nujol | 5000 – 667 cm**(-1) |
| Spectrum | hexachloro-buta-1,3-diene | 5000 – 1250 cm**(-1) |
| Description (Mass Spectrometry) |
| ESI (Electrospray ionisation), Spectrum |
| spectrum |
| spectrum, tandem mass spectrometry, fragmentation pattern |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | aq. HCl | 200 – 260 nm |
| Description (Raman Spectroscopy) |
| Raman |
Route of Synthesis (ROS)
| Conditions | Yield |
| With dihydrogen peroxide for 8h; | 95.7% |
| With air for 13h; Oxidation; | 40% |
| In water at 20℃; for 168000h; light irradiation; | |
| With air In water at 40℃; for 24h; | |
| With fluorone black In methanol; water at 20℃; for 0.0833333h; pH=7.3; Experimental Procedure General procedure: Example 3 An alternative method for the selective detection of homocysteine employs fluorone black, Compound 4. (See FIG. 2.) Following the addition of a thiol (Hcy, Cys, or GSH) to a solution of Compound 4 (1.0×10−5 M) in 70percent MeOH/H2O (phosphate buffer, H2O, pH=7.3), an increase in absorbance occurred at 510 nm at room temperature (analysis after 5 min). The absorbance increase was greatest for Hcy as compared to equimolar amounts of the other two biothiol analytes. Amino acids lacking thiol functionality, such as L-alanine, L-arginine, L-glutamine, glycine, L-lysine, L-methionine, L-serine, and L-threonine, did not produce substantial spectral changes at 510 nm as compared to solutions of Compound 4 without analyte. Note in particular that methionine, which contains a sulfur atom but not a thiol group, did not produce a substantial spectral change at 510 nm. We have discovered that potential interferences may be minimized, and outstanding selectivity achieved, by the addition of a reducing agent such as a phosphine derivative (5 equiv. to analyte in this example). Without wishing to be bound by this theory, our findings suggested a process in which Compound 4 was involved in the redox chemistry of the thiols. 1H NMR studies showed that conversion of homocysteine (the reduced, thiol form, RSH) to homocystine (the oxidized, disulfide form of homocysteine, RSSR) was enhanced in the presence of Compound 4. Additionally, the MALDI mass spectrum of products formed in a solution containing Compound 4 and Hcy exhibited prominent peaks for the sodium salt of glycine, and for the disodium and dipotassium salts of a glycine-derived dimer. Glycine and its dimerization products are known to be termination products of α-amino acid carbon-centered radicals. MALDI TOF MS (anthracene matrix), calculated for glycine sodium salt C2H4NNaO2 (M+Na)+ 97.01. found 96.89; calculated for glycine dimer (2,3-diaminosuccinic acid disodium salt) C4H6Na2N2O4 (M+2Na)+ 192.01. found 193.05; calculated for glycine dimer (2,3-diaminosuccinic acid dipotassium salt) C4H6K2N2O4 (M+2K)+ 223.96. found 223.86. |
Safety and Hazards
| GHS Hazard Statements | NONH for all modes of transport |
| For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: 7-bromo-6-chloro-3-[3-[(2S,3R)-3-hydroxypiperidin-2-yl]-2-oxopropyl]quinazolin-4-one;hydrobromide URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/228694 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | NONH for all modes of transport |
| Store at 2-8℃, seald and away from light | |
| HS Code | 293090 |
| Storage | Store at 2-8℃, seald and away from light |
| Shelf Life | No data available |
| Market Price | USD |
| Use Pattern |
| L-Homocystine CAS# 626-72-2 can maintain the thiolase activity in keratin production; |
| L-Homocystine CAS# 626-72-2 can also restore liver function and also has a wide range of detoxification effects. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Apnoke Scientific Ltd | http://www.apnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |