N-PROPYL ACETATE CAS#: 109-60-4; ChemWhat Code: 1411698
Identification
| Product Name | N-PROPYL ACETATE |
| IUPAC Name | propyl acetate |
| Molecular Structure | 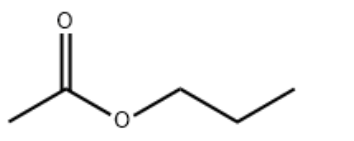 |
| CAS Registry Number | 109-60-4 |
| EINECS Number | 203-686-1 |
| MDL Number | MFCD00009372 |
| Synonyms | Propyl acetate 109-60-4 N-PROPYL ACETATE Acetic acid, propyl ester Propyl ethanoate 1-Acetoxypropane n-Propyl ethanoate Octan propylu Acetic acid n-propyl ester Propylacetate Acetate de propyle normal n-Propyl acetate (natural) Acetic acid propyl ester Propylester kyseliny octove Octan propylu [Polish] n-propanol acetate EINECS 203-686-1 Acetic acid, n-propyl ester UNII-4AWM8C91G6 BRN 1740764 4AWM8C91G6 DTXSID6021901 CHEBI:40116 AI3-24156 Acetate de propyle normal [French] Propylester kyseliny octove [Czech] DTXCID301901 ACETIC ACID,PROPYL ESTER EC 203-686-1 4-02-00-00138 (Beilstein Handbook Reference) PROPYL ACETATE (USP-RS) n-propylacetat n-Propyl ester of acetic acid acetic acid propyl Propyl acetate, N- ACETATE, PROPYL Propyl acetate, 99% PAT (CHRIS Code) Actate de propyle normal CH3COOCH2CH2CH3 Propyl ester of acetic acid PROPYL ACETATE [MI] FEMA NUMBER 2935 SCHEMBL14991 PROPYL ACETATE [FCC] WLN: 3OV1 CHEMBL44857 PROPYL ACETATE [FHFI] PROPYL ACETATE [INCI] Propyl acetate, >=99.5% Propyl acetate, >=98%, FG N-PROPYL ACETATE [HSDB] N-Propyl acetate LBG-64752 Propyl Acetate (Fragrance Grade) Propyl Acetate (Industrial Grade) ACETIC ACID, N-PROPYL ETHER NSC72025 Tox21_202012 MFCD00009372 NA1276 STL280317 AKOS008949448 Propyl acetate, natural, >=97%, FCC, FG n-Propyl acetate [UN1276] [Flammable liquid] n-Propyl acetate [UN1276] [Flammable liquid] Propyl acetate, United States Pharmacopeia (USP) Reference Standard Propyl Acetate, Pharmaceutical Secondary Standard; Certified Reference Material |
| Molecular Formula | C5H10O2 |
| Molecular Weight | 102.13 |
| InChI | InChI=1S/C5H10O2/c1-3-4-7-5(2)6/h3-4H2,1-2H3 |
| InChI Key | YKYONYBAUNKHLG-UHFFFAOYSA-N |
| Canonical SMILES | CCCOC(=O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN107987044 | A process for manufacturing a δ – e lactone method (by machine translation) | 2018 |
| CN105237342 | Method for preparing alcohol through catalytic hydrogenation reduction of carboxylate | 2016 |
| WO2014/144685 | METHODS FOR IDENTIFYING ARTHROPOD REPELLENTS AND ATTRACTANTS, AND COMPOUNDS AND COMPOSITIONS IDENTIFIED BY SUCH METHODS | 2014 |
| US2010/292496 | Process for Production of Alkyl Tin Alkoxide Compound, and Process for Production of Carbonic Acid Ester Using the Compound | 2010 |
Physical Data
| Appearance | Colorless, clear and no mechanical impurities |
| Melting Point, °C |
| -95 |
| -92.5 |
| Boiling Point, °C |
| 101.16 |
| 101.24 |
| 100 – 102 |
| 92 |
| 101.18 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 0.85995 | 44.99 |
| 0.87695 | 29.99 |
| 0.86565 | 39.99 |
| 0.87132 | 34.99 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Partner (Association (MCS)) |
| Sorption diagram | ||
| Rate of adsorption | 20 | dinonyl phthalate |
| Rate of adsorption | 20 | Triton X-305 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | |||
| Chemical shifts, Spectrum | 1H | |||
| Chemical shifts | 1H | chloroform-d1 | 600 | |
| Chemical shifts | 13C | chloroform-d1 | ||
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 26.84 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 26.84 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | ||
| Bands | ||
| Mid IR (MIR), Bands | nujol | |
| Bands | KBr | in the presence of organic compounds |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | H2O, H2SO4 | Ratio of solvents: 66percent | 258 | 5740 |
| Absorption maxima | H2O, NaOH | Ratio of solvents: 0.1N | 232, 290 | 8600, 3120 |
Route of Synthesis (ROS)
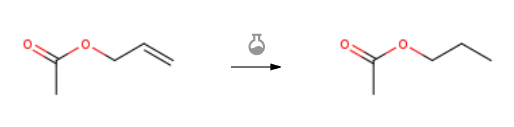
Route of Synthesis (ROS) of N-PROPYL ACETATE CAS 109-60-4
| Conditions | Yield |
| With hydrogen In neat (no solvent) at 30℃; for 5h; Catalytic behavior; Flow reactor; | 100% |
| With hydrogen; 0.5% Pd/C at 80℃; for 100h; Product distribution / selectivity; | 99.3% |
| With hydrogen; 0.3 % Pd/C In lithium hydroxide monohydrate at 40℃; under 6000.6 Torr; Product distribution / selectivity; | 99.97% |
| Experimental Procedure Third distillation process:21 parts by mass of the distillate (Yl) obtained in the second distillation process of Example 1 were supplied to a third distillation column, and distilled under the following conditions: top pressure of 5 kPaG, bottom pressure of 45 kPaG, top temperature of 105°C, bottom temperature of 135°C, reflux ratio of 1.7, and evaporation rate of 96% by mass. A distillate (Zl) containing high-purity allyl acetate was obtained from the top of the distillation column. The concentration of water in the obtained distillate (Zl) was 30 ppm, the concentration of allyl acetate was 99.74% by mass, the concentration of acetic acid was less than 5 ppm, and the concentration of allyl acrylate was less than 5 ppm.[0088]Hydrogenation process:Next, the distillate (Zl) was subjected to a hydrogenation reaction in the same manner as in Example 1, thus obtaining n-propyl acetate.With regard to the obtained n-propyl acetate, the conversion rate of allyl acetate was 99.9%, the selectivity of l-propenyl acetate was 0.21%, the selectivity of acetic acid was 0.25%, and the yield of n-propyl acetate was 99.97%. In addition, theconcentration of propyl propionate in the obtained reaction liquid was less than 5 ppm. | 99.97% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H225: Highly Flammable liquid and vapor [Danger Flammable liquids] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H336: May cause drowsiness or dizziness [Warning Specific target organ toxicity, single exposure; Narcotic effects] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264+P265, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P319, P337+P317, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 102.133 |
| logP | 1.022 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Quantitative Results | |||
| 1 of 203 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | PROCESS FOR PRODUCING HYDROGENATED ESTER, HYDROGENATION CATALYST FOR USE THEREIN, AND PROCESS FOR PRODUCING THE CATALYST | ||
| 2 of 203 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | High affinity ligands for nociceptin receptor ORL-1 | ||
| 3 of 203 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | SUBSTITUTED PIPERIDINES AS MELANOCORTIN RECEPTOR AGONISTS | ||
| 4 of 203 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | AN EFFICIENT PROCESS FOR THE MANUFACTURE OF (E)-ENTACAPONE POLYMORPHIC FORM A | ||
| 5 of 203 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Tc-labeled arylpiperazine derivatives for imaging serotonin receptor | ||
| 10 of 10 | Results | effect on phosphatidylcholine secretion in primary cultures of rat type II pneumocytes |
| Use Pattern |
| N-PROPYL ACETATE CAS#: 109-60-4 is a versatile organic solvent with good solubility and volatility. It is used to dissolve various organic compounds, including resins, coatings, paints, adhesives, and coating materials. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to [email protected] |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

